Figure 3. Model for suppression of KV channel expression and function in diabetes.
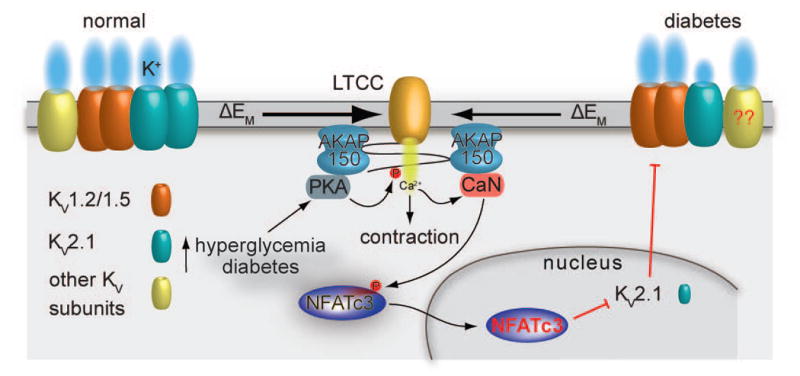
PKA-mediated phosphorylation of activation of serine 1928 induces potentiation of LTCCs in response to hyperglycemia and during diabetes (62). This leads to increased global Ca2+ influx and contraction. The increase in Ca2+ influx promotes activation of the AKAP150-targeted calcineurin, which dephosphorylates NFATc3 and allows its nuclear translocation where the transcription factor can suppress the expression of KV2.1 (but not KV1.2 and KV1.5) subunits. This reduction in KV2.1 expression and function decreases voltage-gated K+ currents and the negative feedback membrane potential hyperpolarization, thus leading to membrane potential depolarization, further Ca2+ influx through LTCCs, vascular smooth muscle contraction and enhanced vascular tone. Whether changes in KV7.X subunits during diabetes proceed through the calcineurin/NFATc3 signaling pathway and similar changes occur in the human vasculature require further investigation. Illustration of the interaction between AKAP150 and LTCC does not necessarily reflect the native interaction between these proteins. The cartoon was drawn as such for simplicity.
