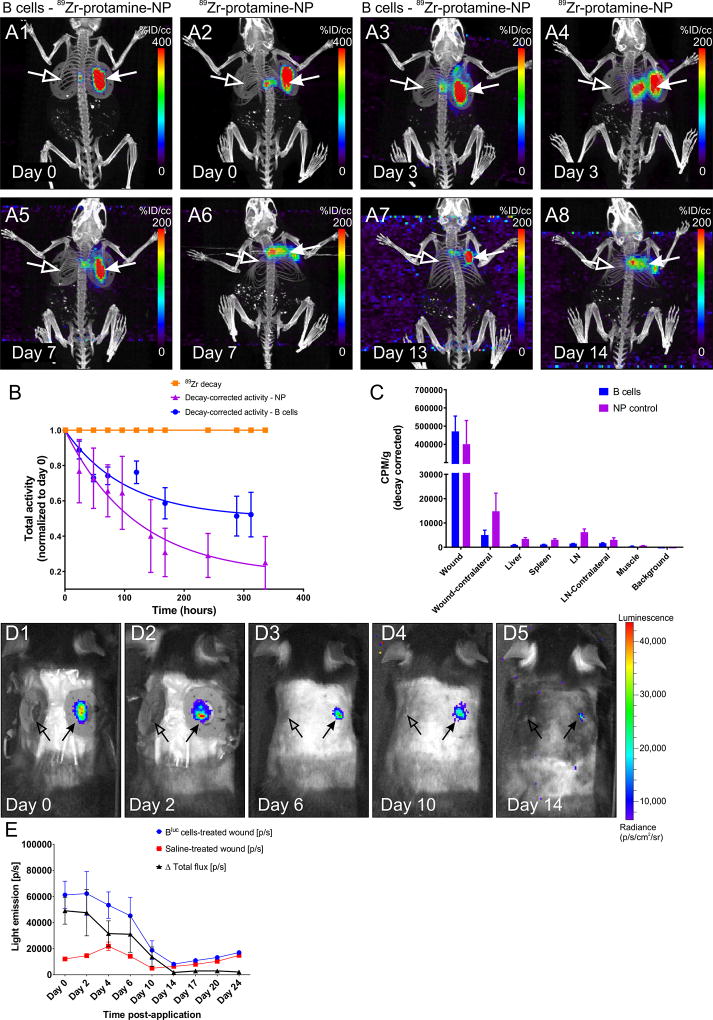
Figure 5. PET/CT tracking of B cells after application to the wound bed.
A1–8 Representative maximum intensity projection (MIP) images of PET/CT scans from a WT animal at various intervals after application of 89Zr-protamine-nanoparticle-labeled B cells or equal amounts of nanoparticle control. Note the highly restricted localization of the radioactive signal to the treated wound bed and edges (arrows) in the cell-treated condition (A1, A3, A5, A7), while substantial diffusion of the radioactivity was observed in the absence of cells (A2, A4, A6, A8; nanoparticle alone). Virtually no signal can be detected in the contralateral wound (open arrows) or in other organs of the body at this signal intensity. B. 89Zr-decay-corrected radioactivity at the treated wound site (n = 4 animals per condition) indicates that the leakage of activity from the wound site is much more pronounced if the nanoparticle is applied in the absence of cells. 89Zr decay is set at 1 and shown for comparison. C. Gamma counter measurements of residual radioactivity at the end point of the experiments in various organs confirms the localization of the signal in the B cell treated wounds and the increased distribution to liver and lymph nodes after free nanoparticle application. CPM/g = counts per minute per gram tissue. D1–5. B cell survival at the wound site. Intravital imaging of a WT mouse at multiple time points after application of Bluc cells (right-side wound, arrow) or saline control (left-side wound, open arrow). E. Light emission from the wound site treated with luciferase-expressing B cells (n = 6 animals per condition) indicates that the cells survive in situ up to approximately 14 days after application, with numbers of viable cells decreasing markedly after day 6. [p/s] = photons per second.