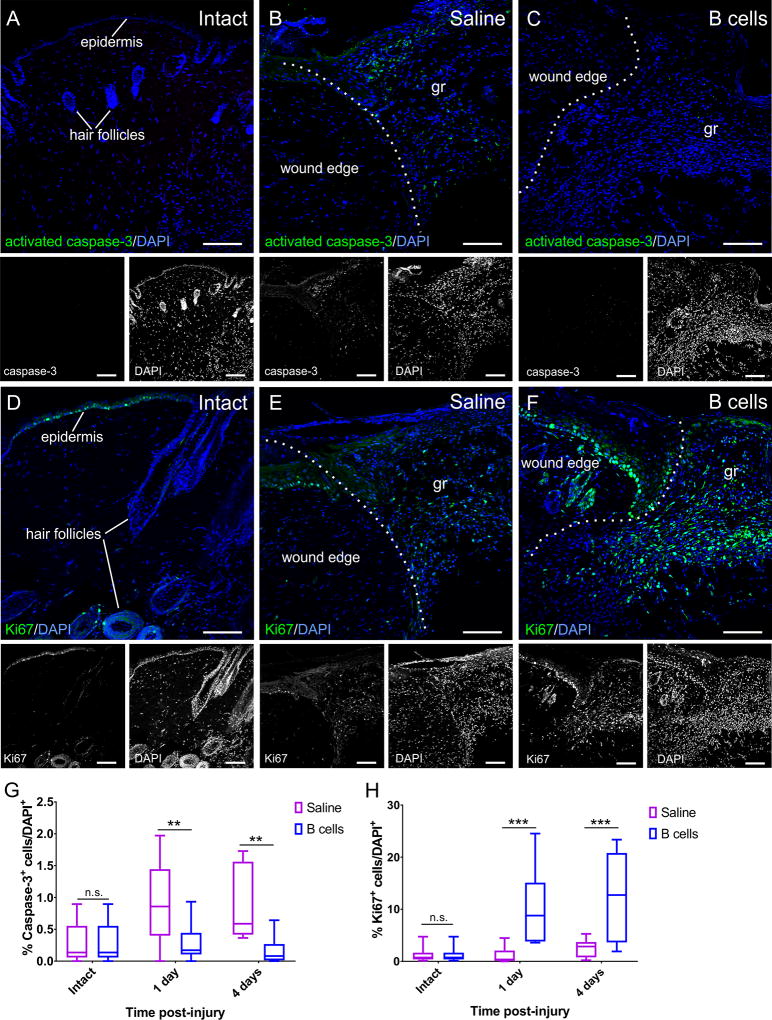
Figure 6. B cell application at the time of injury alters the rates of apoptosis and cell proliferation in the wound bed and edges.
A–C Confocal images of transverse sections through the wound edge collected at 4 days post-injury are shown immunolabeled against the apoptosis marker activated caspase-3. Cell nuclei are counterstained with DAPI. While very few caspase-3-positive cells can be found in intact skin (A), injury induces high levels of apoptosis (B), which is significantly reduced in wounds treated with B cells (C). Scale bars, 100 µm. D–F. Confocal images of transverse sections through the wound edge at 4 days post-injury immunolabeled against the cell proliferation marker Ki67. Cell nuclei were counterstained with DAPI. Cell proliferation in the intact skin is mostly restricted to the stem cells in the hair follicles and the epidermis (D). Injury induces a regenerative response, elevating numbers of proliferating cells which invade the wound bed (E), and this response appears greatly amplified in wounds treated with B cells (F). gr = granulation tissue in the wound bed. Scale bars, 100 µm. G–H. Quantitative analysis of apoptotic caspase-3-immunopositive cells (G) and proliferating Ki67-immunopositive cells (H) in the wound bed and edges at 1 and 4 days post-injury. Scale bars, 100 µm. At least 3 fields from 3 distinct sections were analyzed per animal; at least 3 animals were analyzed per condition and time point. Statistical significance was assessed by two-way ANOVA, followed by Tukey’s multiple comparisons test.** = p < 0.01; *** = p < 0.001; **** = p < 0.0001.