Figure 4. cGAS driven signaling licenses non-canonical inflammasome and RPE degeneration.
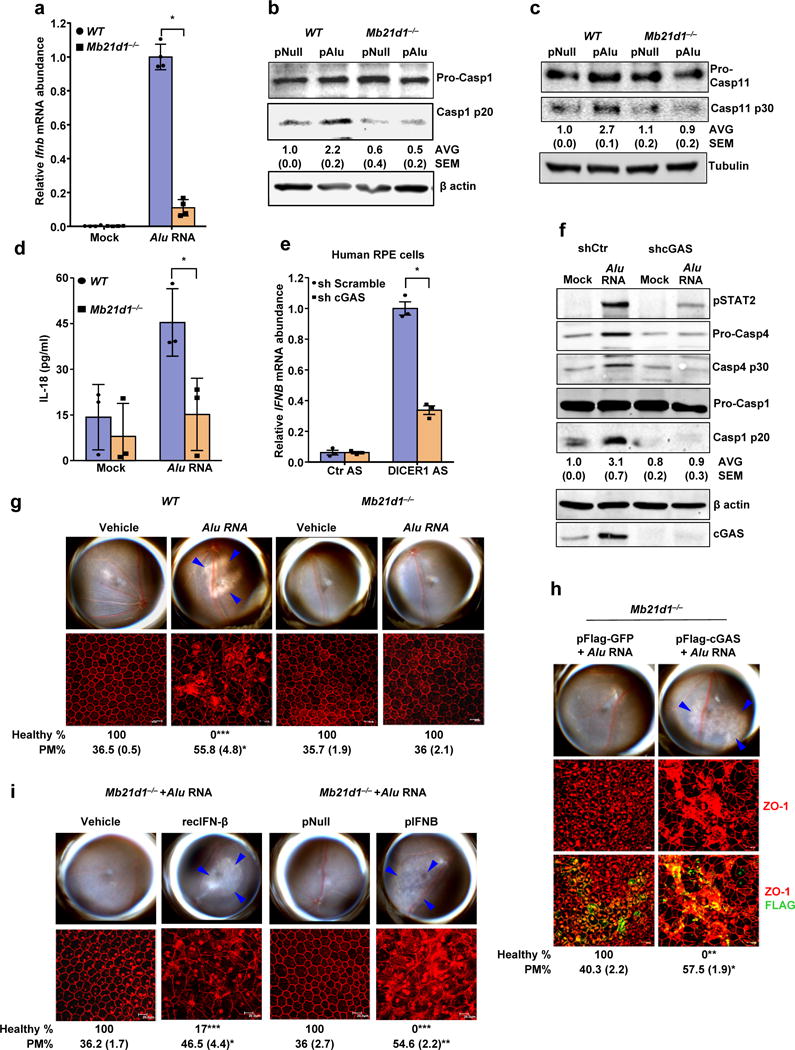
(a) Relative abundance of Ifnb mRNA in WT and Mb21d1−/− mouse RPE cells mock-transfected or transfected with Alu RNA. Data presented are mean ± SEM; n = 4 cell culture replicates; *P = 0.0001, two-tailed t test. (b) Immunoblots of pro-caspase-1 (pro-Casp1) and the p20 cleavage product of caspase-1 (Casp1 p20) in WT and Mb21d1−/− mouse RPE cells transfected with Alu expression plasmid (pAlu) or empty vector control (pNull). (c) Immunoblots of pro-caspase-11 (pro-Casp1) and the p30 cleavage product of caspase-1 (Casp11 p30) in WT and Mb21d1−/− mouse RPE cells transfected with Alu expression plasmid (pAlu) or empty vector control (pNull). (d) IL-18 secretion by WT and Mb21d1−/− mouse RPE cells mock transfected or transfected with Alu RNA. Data presented are mean ± SD; n = 3 independent experiments; *P = 0.032, two-tailed t test. (e) Relative abundance of IFNB mRNA in control (sh Scramble) or cGAS shRNA knockdown human RPE cells transfected with or DICER1 or control (Ctr) anti-sense oligonucleotides (AS). Data presented are mean ± SEM; n = 3 cell culture replicates; *P = 0.0002, two-tailed t test. (f) Immunoblot of phosphorylated STAT2 (pSTAT2); pro-caspase-4 and casp4 p30; pro-caspase-1 and p20 cleavage casp1 p20 in control (sh Scramble) or cGAS shRNA knockdown human RPE cells mock-transfected or transfected with Alu RNA. Knockdown efficiency of cGAS is shown by cGAS immunoblot and tubulin was used as a loading control. (g) Fundus photographs and immunofluorescence staining of zonula occludens-1 (ZO-1) on RPE flat mounts of WT (n = 6 eyes) and Mb21d1−/− (n = 8 eyes) mice subretinally injected with vehicle or Alu RNA. (h) Fundus photographs and immunofluorescence staining of zonula occludens-1 (ZO-1) on RPE flat mounts of Mb21d1−/− (n = 7 eyes) mice reconstituted by in vivo transfection of cGAS expression plasmid (pFlag-cGAS) or control GFP expression plasmid (pFlag-GFP), subretinally injected with Alu RNA. (i) Fundus photographs and immunofluorescence staining of zonula occludens-1 (ZO-1) on RPE flat mounts of Mb21d1−/− mice subretinally co-administered Alu RNA with recombinant IFN-β (n = 6 eyes), vehicle control (n = 6 eyes), IFN-β expression plasmid (pIFNB; n = 5 eyes) or empty vector control (pNull; n = 5 eyes). For all immunoblots, cropped gel image of bands of interest of representative immunoblots of three independent experiments and densitometric analysis (mean (SEM)) are shown. In g, h and i, binary (Healthy %) and morphometric (PM, polymegethism (mean (SEM)) quantification of RPE degeneration are shown (Fisher’s exact test for binary; two-tailed t test for morphometry; *P < 0.05; **P < 0.01; ***P < 0.001). Loss of regular hexagonal cellular boundaries in ZO-1 stained flat mounts is indicative of degenerated RPE. The degenerated retinal area is outlined by blue arrowheads in the fundus images.
