Figure 1. CLIP170 induces ubiquitination and degradation of TIRAP.
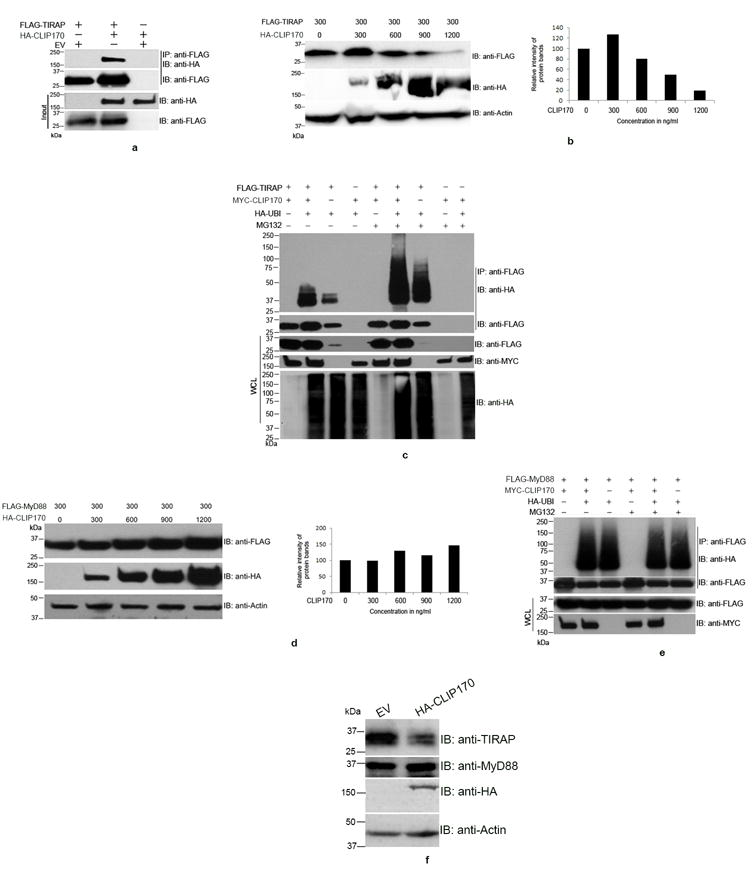
(a) CLIP170 interacts with TIRAP. HEK293T cells were co-transfected with equal amounts of HA-CLIP170 and FLAG-TIRAP plasmids. Twenty four hours post-transfection, cells were lysed and FLAG-TIRAP was immunoprecipitated using anti-FLAG antibody followed by immunoblotting. The blot was probed with anti-HA antibody to detect the co-immunoprecipitated HA-CLIP170 followed by detection of FLAG-TIRAP by anti-FLAG antibody. The whole cell lysates were also subjected to immunoblotting followed by immuno-detection of HA-CLIP170 and FLAG-TIRAP. (b) CLIP170 promotes degradation of TIRAP. HEK293T cells were co-transfected with FLAG-TIRAP and increasing concentrations of HA-CLIP170 plasmids. Twenty four hours post-transfection, cells were lysed and subjected to immunoblotting. The blot was probed with anti-FLAG and anti-HA antibodies to detect FLAG-TIRAP and HA-CLIP170, respectively. Actin served as the loading control. Right panel of the immunoblot shows the densitometry analysis of FLAG-TIRAP bands normalized to actin; (c) CLIP170 induces ubiquitination of TIRAP. HEK293T cells were co-transfected with various combinations of FLAG-TIRAP, MYC-CLIP170 and HA-Ubiquitin as indicated. Twenty four hours post-transfection, cells were treated with 20 μm MG132 for 4 h as indicated in the figure. Cells were then lysed and FLAG-TIRAP was immunoprecipitated followed by immunoblotting. The blot was probed with anti-HA antibody to detect the HA-Ubiquitin-conjugated FLAG-TIRAP. CLIP170 enhanced the ubiquitination of CLIP170 in MG132 treated or untreated cells. Similar concentrations of FLAG-TIRAP and MYC-CLIP170 resulted in accumulation of ubiquitinated FLAG-TIRAP in the immunoprecipitated samples. (d) CLIP170 did not induce degradation of MyD88. HEK293T cells were co-transfected with 300 ng of FLAG-MyD88 and increasing concentrations of HA-CLIP170. Twenty four hours post-transfection, cells were lysed and subjected to immunoblotting. The blot was probed with anti-FLAG and anti-HA antibodies to detect FLAG-MyD88 and HA-CLIP170, respectively. Degradation of MyD88 was not observed with increasing concentrations of HA-CLIP170. Right panel of the immunoblot shows the densitometry analysis of FLAG-MyD88 bands normalized to actin; (e) CLIP170 did not promote the ubiquitination of FLAG-MyD88. Ubiquitination assay was performed as described before with the FLAG-MyD88 as the substrate. Ubiquitination status of MyD88 was not affected by CLIP170; (f) CLIP170 induces degradation of endogenous TIRAP. RAW264 cells were transfected with EV or HA-CLIP170 followed by detection of endogenous TIRAP and MyD88 using anti-TIRAP and anti-MyD88 antibodies, respectively. Actin served as the loading control. Immunoblots are representative of two independent experiments. EV: Empty vector; IP: immunoprecipitation; IB: immunoblotting.
