Abstract
Methadone is used for medication-assisted treatment of heroin addiction during pregnancy. The neurodevelopmental outcome of children with prenatal methadone exposure can be sub-optimal. We tested the hypothesis that brain development is altered among newborn infants whose mothers were prescribed methadone.
20 methadone-exposed neonates born after 37 weeks' postmenstrual age (PMA) and 20 non-exposed controls underwent diffusion MRI at mean PMA of 39+ 2 and 41+ 1 weeks, respectively. An age-optimized Tract-based Spatial Statistics (TBSS) pipeline was used to perform voxel-wise statistical comparison of fractional anisotropy (FA) data between exposed and non-exposed neonates.
Methadone-exposed neonates had decreased FA within the centrum semiovale, inferior longitudinal fasciculi (ILF) and the internal and external capsules after adjustment for GA at MRI (p < 0.05, TFCE corrected). Median FA across the white matter skeleton was 12% lower among methadone-exposed infants. Mean head circumference (HC) z-scores were lower in the methadone-exposed group (− 0.52 (0.99) vs 1.15 (0.84), p < 0.001); after adjustment for HC z-scores, differences in FA remained in the anterior and posterior limbs of the internal capsule and the ILF. Polydrug use among cases was common.
Prenatal methadone exposure is associated with microstructural alteration in major white matter tracts, which is present at birth and is independent of head growth. Although the findings cannot be attributed to methadone per se, the data indicate that further research to determine optimal management of opioid use disorder during pregnancy is required. Future studies should evaluate childhood outcomes including infant brain development and long-term neurocognitive function.
Keywords: Prenatal, Methadone, Brain, Neonate, MRI, Opioid
Highlights
-
•
Prenatal methadone exposure is associated with atypical white matter development.
-
•
Reduced FA in the white matter skeleton is apparent soon after birth.
-
•
Polydrug use among cases limits causal inference.
-
•
Infant brain development should be evaluated in studies of opioid use in pregnancy.
1. Introduction
Globally, in 2015 there were estimated to be 17.7million past-year users of heroin or opium, and increased heroin use is a major driver of the current opioid epidemic (World Drug Report, 2017). Pregnant women with opioid use disorder (OUD) due to heroin are recommended medication-assisted treatment (MAT) with an alternative opioid (usually methadone or buprenorphine) because treatment is associated with improved use of antenatal services, reduced use of heroin during pregnancy and reduced preterm delivery. Fetal benefits of MAT include improved growth and lower risk of intrauterine death (American College of Obstetricians and Gynecologists, 2017).
Methadone is a synthetic long acting μ-opioid agonist, which crosses the placenta freely, thereby exposing the developing fetus to exogenous opioid at a critical period of brain development. Pre-clinical studies suggest that prenatal methadone exposure may modify developing dopaminergic, cholinergic and serotonergic systems, and alter myelination. Antenatal exposure to the drug has behavioral consequences including depression, anxiety, and impaired learning, memory and social function (Chen et al., 2015, Robinson et al., 1996, Vestal-Laborde et al., 2014, Wong et al., 2014). In humans, prenatal methadone exposure is associated with increased incidence and severity of neonatal abstinence syndrome (NAS) (Wilson et al., 1981, Zelson et al., 1973) compared with heroin exposure, and with altered visual maturation in childhood (McGlone et al., 2013a, McGlone et al., 2008, Whitham et al., 2010). These observations raise the possibility that prenatal methadone exposure may modify early brain development; however, the possible role of confounding by postnatal events, including pharmacotherapy with opioid for NAS and environmental factors, leaves uncertainty about the impact of prenatal methadone exposure on the developing brain.
Diffusion MRI (dMRI) is an established technique for studying brain development in early life. It provides objective measures of white matter microstructure that are sensitive to atypical developmental and injurious processes in the perinatal period, and which correlate with neurodevelopmental outcome in childhood (Counsell et al., 2014). Specifically, fractional anisotropy (FA) is a voxel-wise measure of the directional dependence of water molecule diffusion in tissue which is influenced by fiber density, axonal diameter and myelination, thereby enabling inference about underlying tissue microstructure. Tract-based Spatial Statistics (TBSS) enables unbiased group-wise analysis of FA volumes derived from dMRI data (Ball et al., 2010, Smith et al., 2006). It has been applied to neonatal dMRI to map microstructural change in white matter tracts of preterm infants at term equivalent age (Anjari et al., 2007), to identify clinical risk factors for altered brain development (Anblagan et al., 2016, Ball et al., 2010, Boardman et al., 2014), and to investigate neuroprotective treatment strategies in randomized clinical trials (Azzopardi et al., 2016, O'Gorman et al., 2015, Porter et al., 2010).
Based on the harmful effects of prenatal methadone exposure on neural systems and abnormal behavioral outcomes in pre-clinical models; and on human studies which suggest a modifying effect of prenatal methadone on postnatal behavior and development, we hypothesized that white matter development of neonates would be altered in neonates exposed to methadone in utero. We used TBSS to examine risks associated with prenatal methadone exposure, while minimizing the role of confounding by postnatal events and drug exposures.
2. Methods and materials
2.1. Participants
The study was conducted according to the principles of the Declaration of Helsinki and ethical approval was obtained from the UK National Ethics Service (South East Scotland Research Ethics Committee 02, 14/SS/1106). Written informed parental consent was obtained for all participants. The study group consisted of infants > 37 weeks' postmenstrual age (PMA) whose mothers had been prescribed methadone during pregnancy for the treatment of OUD (cases) and a comparator group of healthy infants born at > 37 weeks' PMA whose mothers did not use opioids (controls).
Mothers of cases were identified through a specialist antenatal clinic for pregnant women with substance misuse. All cases were born at the Royal Infirmary of Edinburgh between February 2015 and April 2017 and underwent MRI brain scanning at the Clinical Research Imaging Centre, University of Edinburgh. The controls were selected, based on age matching, from a previously described group of healthy term neonates recruited as part of a study of typical brain development (Blesa et al., 2016) (South East Scotland Research Ethics Committee 02, 13/SS/0143). For cases and controls, exclusion criteria were congenital infection or chromosomal abnormalities, or any implanted medical device.
Clinical and demographic information was extracted from the mother and infant clinical records. Birth weight and head circumference (HC) were described in terms of z-score for week of gestational age, calculated using INTERGROWTH-21st reference standards (Villar et al., 2014). The Scottish Index of Multiple Deprivation (SIMD) was used to characterize deprivation. The SIMD is the official Government tool used to identify areas of deprivation: it divides Scotland into around 6505 areas each containing around 350 households and assigns an index to each area based on multiple measures of deprivation. The data are ranked from most to least deprived and are presented as deciles.
2.2. Ascertainment of maternal drug use
Details of methadone use, tobacco smoking, alcohol intake, and use of non-prescribed drugs were ascertained from medical records (including prescription charts), biological screening samples when these were performed as part of clinical care, and maternal interview at the time of delivery (V.M.)
2.3. MRI acquisition
MRI was performed on a Siemens Magnetom Verio 3T system (Siemens Healthcare Gmbh, Erlangen, Germany) using a 12-channel matrix phased array head coil. All infants were scanned axially to acquire: 3D T1-weighted MPRAGE volume (1 mm3 resolution), T2-weighted STIR (0.9 mm3 resolution), T2-weighted FLAIR (1 mm3 resolution), and diffusion MRI (dMRI) (11 T2- and 64 diffusion encoding direction (b = 750 s/mm2) single-shot spin-echo echo planar imaging (EPI) volumes with 2 mm isotropic voxels, TE = 106 ms and TR = 7300 ms. Images were reported by a pediatric radiologist with experience in neonatal MRI (AQ), according to the system described by Woodward et al. (Woodward et al., 2006), with the modification for grey matter scores proposed by Leuchter et al. (Leuchter et al., 2014).
MRI was performed in the neonatal period during natural sleep, without sedation. A neonatologist was present for the duration of each MRI scan, and the infant had continuous oxygen saturation and heart rate monitoring. For acoustic protection, flexible earplugs and neonatal earmuffs (MiniMuffs, Nat's Medical Inc., CA) were used.
2.4. Tract-based spatial statistics
DMRI data were preprocessed using FSL tools (FMRIB, Oxford, UK; http://www.ndcn.ox.ac.uk/divisions/fmrib). This included brain extraction, and removal of bulk infant motion and eddy current induced artefacts by registering the diffusion-weighted volumes to the first T2-weighted EPI volume for each subject. Using DTIFIT, FA volumes were generated for every subject. Diffusion volumes were assessed visually and were excluded if there was motion corruption.
TBSS analysis was performed using a pipeline optimized for neonatal dMRI data (Ball et al., 2010). An average FA volume and mean FA skeleton (thresholded at FA > 0.15) were created from the aligned data. Statistical comparison between groups with and without exposure to methadone during pregnancy was performed with FSL's Randomise using a general linear univariate model, with GA at image acquisition and HC z-score at image acquisition listed as covariates. All FA data were subject to family-wise error correction for multiple comparisons following threshold-free cluster enhancement (TFCE) and are shown at p < 0.05 (Smith and Nichols, 2009).
2.5. Statistics
Student's t-test or the Mann-Whitney test was used to investigate differences in clinical and demographic variables between infants exposed to methadone (n = 20) and those not exposed (n = 20) and chi-squared or Fisher's exact test was used to compare proportions. Statistical analysis was performed using SPSS v22.0 (SPSS Inc., Chicago, IL).
3. Results
3.1. Participants
Conventional structural and dMRI data amenable to TBSS analysis were acquired from 40 neonates: 20 cases (10 female), who were exposed to prenatal methadone, and 20 unexposed controls (7 female). Table 1 summarizes maternal and infant characteristics.
Table 1.
Maternal and infant characteristics of participants. BMI, body mass index; SIMD, Scottish Index of Multiple Deprivation; PMA, postmenstrual age; HC, head circumference.
| Methadone |
Control |
p value | |
|---|---|---|---|
| n = 20 | n = 20 | ||
| Maternal characteristics | |||
| Mean age (range)/years | 30 (23–41) | 30.9 (19–39) | 0.56 |
| Mean BMI (range) | 25.8 (21–41) | 23.2 (19–39) | 0.08 |
| Median SIMD decile (Interquartile range) | 3 (2–5) | 8 (6–10) | 0.012 |
| Infant characteristics | |||
| Mean PMA at birth (range)/weeks | 38+ 5 (37+ 1–41+ 0) | 39+ 1 (37+ 2–41+ 3) | 0.32 |
| Gender (M:F) | 10:10 | 13:7 | 0.52 |
| Mean birth weight (range)/g | 2721 (2150–3440) | 3349 (2346–4550) | < 0.01 |
| Mean birth weight z-score (sd) | − 1.062 (0.68) | 0.443 (0.86) | < 0.01 |
| Median (range) postnatal age at scan/days | 3 (1 to 21) | 13 (5 to 29) | 0.005 |
| Mean PMA at scan (range)/weeks | 39+ 2 (37+ 2–41+ 4) | 41+ 1 (39+ 0–42+ 2) | 0.004 |
| Mean HC at scan (range)/cm | 33.1 (31.2–35.0) | 35.9 (32.6–37.4) | < 0.01 |
| Mean HC z-score (sd) | − 0.523 (0.986) | 1.146 (0.837) | < 0.01 |
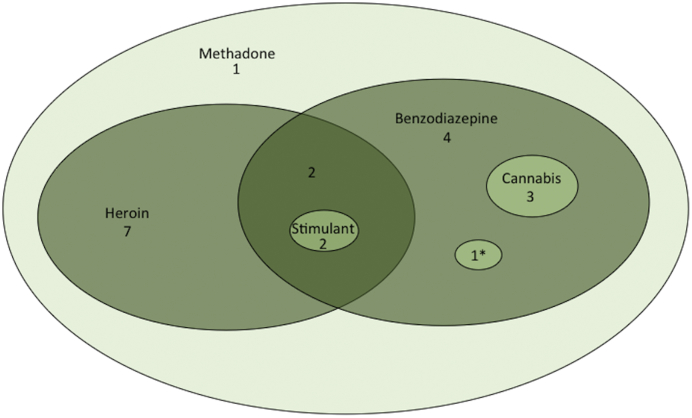
The mean methadone dose prescribed at pregnancy booking was 55 mg/day (range 0–160) and the mean dose at delivery was 70 mg/day (range 8–160). Nineteen (95%) of the women prescribed methadone smoked tobacco, one reported drinking excessive alcohol (4 units/day at booking), and nineteen women had illicit or prescribed polydrug use (Fig. 1). Additional prescribed medications included paroxetine (n = 1), mirtazapine (n = 1), gabapentin (n = 2), and pregabalin (n = 1).
Fig. 1.
Euler diagram indicating prenatal drug exposures. Stimulant includes cocaine and amphetamine; *codeine phosphate and tramadol.
None of the cases had neonatal encephalopathy, seizures or hypoglycemia. The mean arterial cord pH of the group was 7.26 (range 7.16–7.36). Three cases had minor congenital anomalies (1 hypospadias, 1 cleft lip, 1 fixed bilateral talipes). One methadone exposed infant required admission to the Neonatal Unit for treatment of transient tachypnoea of the newborn.
No infant had received pharmacological treatment for NAS at the time of image acquisition and none of the control group was exposed prenatally to opioid drugs.
3.2. Magnetic Resonance Imaging
None of the cases or controls had features consistent with injury to white matter or grey matter on conventional structural T1- or T2-weighted structural MRI. Four cases had mild enlargement of the lateral ventricular system; 1 case had asymmetric myelination of the posterior limb of the internal capsule (but had developed symmetric myelination on repeat MRI four weeks later); and no case had abnormalities in brainstem, cerebellum, deep or cortical grey matter, or extracerebral space. Two (10%) methadone exposed infants had developmental venous anomalies: one consisted of an area of low T2-weighted signal in the left peritrigonal white matter (most likely haemosiderin), with a curvilinear vessel extending peripherally and draining into the superior anastamotic vein of Trolland, which continues up to the superior sagittal sinus; and the second was characterized by an area of low T2-weighted signal in the right peritrigonal white matter with a low T2-weighted signal vessel that drains toward the choroid plexus.
3.3. White matter correlates of prenatal methadone exposure
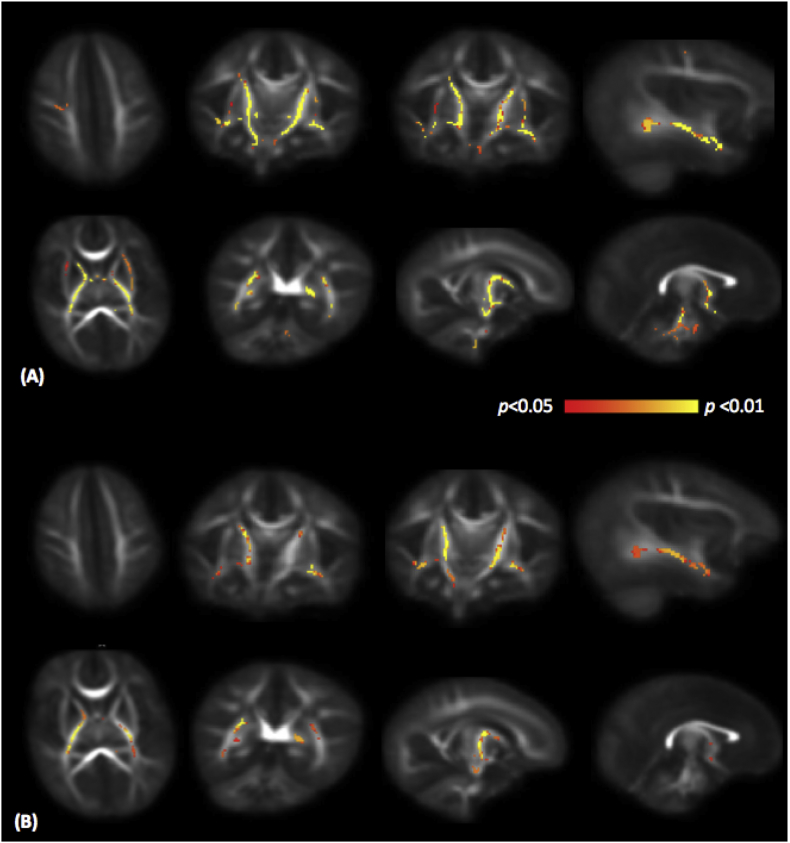
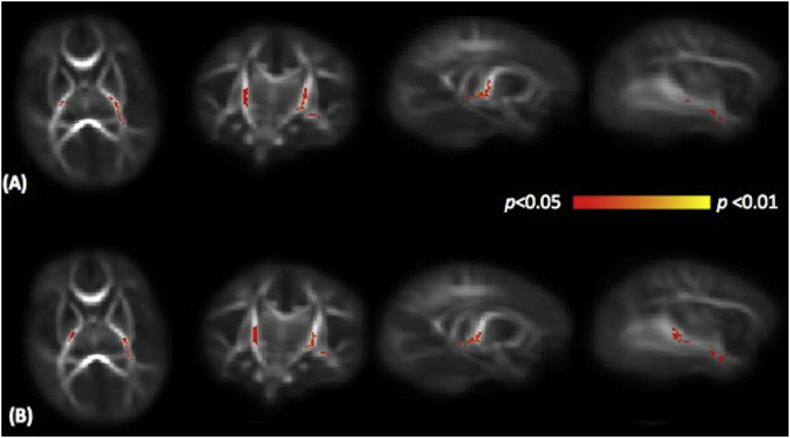
Methadone-exposed neonates had decreased FA within the centrum semiovale, inferior longitudinal fasciculi (ILF), and the internal and external capsules after adjustment for PMA at MRI (p < 0.05, TCFE corrected) (Fig. 2A). Mean HC z-scores were lower in the methadone exposed group (− 0.52 (0.99) vs 1.15 (0.84), p < 0.001). After adjustment for HC z-scores, differences in FA remained in the anterior and posterior limbs of the internal capsule and the ILF (Fig. 2B). Radial diffusivity was increased in internal capsule and inferior longitudinal fasciculus in neonates with prenatal methadone exposure (Fig. 3). There were no differences in mean or axial diffusivities between groups.
Fig. 2.
Mean FA map of the subjects in transverse, coronal and sagittal planes. Voxels with significantly lower FA in neonates with prenatal methadone exposure are shown in yellow-red color scale. Fig. 2A shows results adjusted for PMA at scan, and Fig. 2B shows results adjusted for PMA at scan and head circumference z-score. FA, fractional anisotropy.
Fig. 3.
Mean RD map of the subjects in transverse, coronal and sagittal planes. Voxels with significantly higher RD in neonates with prenatal methadone exposure are shown in yellow-red colour scale. Fig. 3A shows results adjusted for PMA at scan, and Fig. 3B shows results adjusted for PMA at scan and head circumference z-score. RD, radial diffusivity.
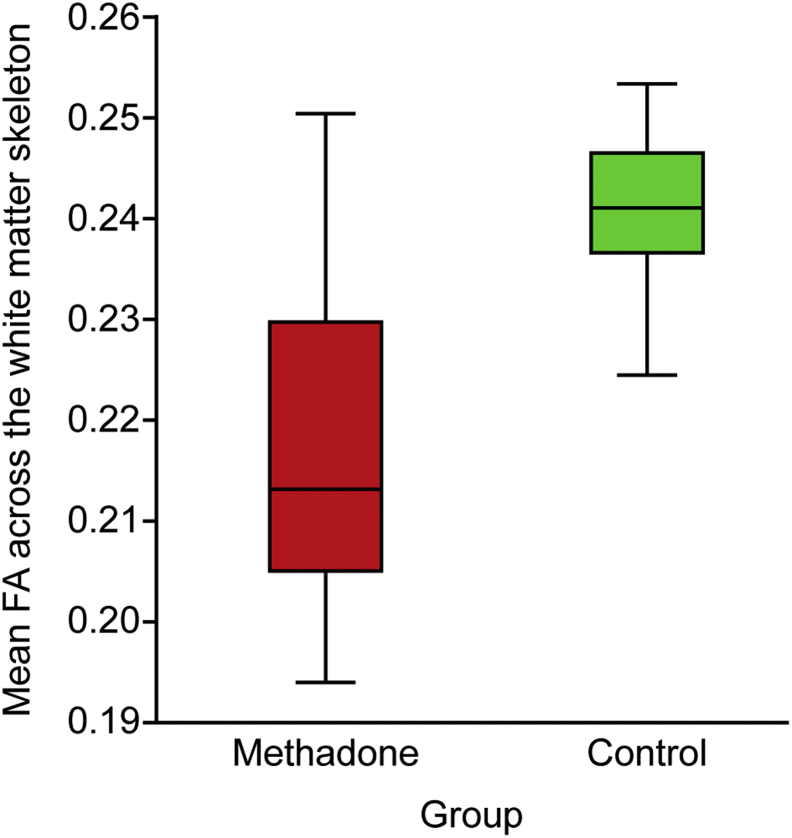
Median FA across the white matter skeleton was 12% lower among methadone-exposed infants (Fig. 4).
Fig. 4.
Mean FA across the white matter skeleton of neonates with prenatal methadone exposure compared with unexposed controls. FA, fractional anisotropy.
4. Discussion
These data show that prenatal exposure to methadone is associated with altered microstructure in major white matter tracts of the newborn brain, independent of head growth. Children whose mothers take methadone during pregnancy are at increased risk of neurodevelopmental impairment, behavioral difficulties, and visual problems, but study designs have left uncertainty about the role of confounding by prematurity, postnatal opioid exposure for treatment of NAS, and environmental factors, in mediating adverse outcomes(Hans and Jeremy, 2001, Hunt et al., 2008, Konijnenberg and Melinder, 2015, McGlone et al., 2014, McGlone and Mactier, 2015, Rosen and Johnson, 1985, van Baar, 1990, Wilson, 1989). An association between prenatal methadone exposure and reduced somatic and head growth is documented (Mactier et al., 2014) but to our knowledge, this is first study to demonstrate brain tissue effects present around the time of birth after methadone exposure in utero.
We used TBSS to investigate brain development because of its sensitivity to group-wise differences in FA when used to survey the entire white matter skeleton (Ball et al., 2013). FA is a robust marker of tract microstructure that reflects fiber density, axonal diameter, wrapping by pre-myelinating oligodendrocytes and myelination. Therefore, these data suggest that neonates exposed to methadone in utero have less coherently organized and more immature fiber tracts compared to controls. Furthermore, correspondent increases in radial diffusivity without changes in axial diffusivity imply that abnormal myelination may contribute to altered FA among the cases. Since neonatal FA values in major white matter tracts correlate with later neurodevelopment, the findings may explain the prevalence of neurobehavioral problems seen in children with prenatal methadone exposure.
Quantitative MRI techniques have identified specific vulnerabilities of the developing brain to psychoactive drugs. Midazolam exposure during neonatal intensive care of preterm infants is associated with attenuated hippocampal growth (Duerden et al., 2016), and functional connectivity of the amygdala–frontal and thalamic networks is altered in neonates with prenatal cocaine exposure (Salzwedel et al., 2016, Salzwedel et al., 2015). In a preliminary study, Walhovd and colleagues reported higher mean diffusivity in the superior longitudinal fasciculus of 13 methadone-exposed cases compared to 7 controls, but infants were scanned at mean age of 3 weeks after birth and 85% of the cases had been treated with morphine for NAS, which limits inference about the effects of prenatal opioid exposure (Walhovd et al., 2012). Two (10%) cases had developmental venous anomalies (DVA), which was higher than expected based on estimated prevalence of 1.5% in neonates (Brinjikji et al., 2017). These did not occur in the cases exposed to cocaine, which is known to be associated with central nervous system vascular anomalies (Frank et al., 1999); therefore the possibility that prenatal methadone exposure is associated with CNS vascular malformation warrants further study.
All of our cases had been exposed to once daily dosing with methadone. Although the benefits of MAT with an opioid substitute during pregnancy are unequivocal (ACOG, 2017), there is no consensus regarding the optimal dosing regimen for methadone, or the role of buprenorphine as an alternative substitute. A single daily dose of methadone is commonly prescribed, but some authors suggest that accelerated metabolism of methadone by physiological induction of CYP450 enzymes during pregnancy might predispose women and fetuses to daily withdrawal stress and risk of relapse to illicit drugs (Bogen et al., 2013, McCarthy et al., 2017, McCarthy et al., 2015). Studies that support the use of divided dosing to minimize fluctuations in serum concentration report favorable effects on maternal symptoms of withdrawal, and on fetal neurobehavior and NAS prevalence (Jansson et al., 2009, McCarthy et al., 2015, Wittmann and Segal, 1991), but no study has evaluated the impact of dosing regimen on fetal/neonatal brain development or long-term outcome. Monitoring of maternal plasma methadone concentration during the peripartum period with dose titration to keep levels within the maternal therapeutic range has been suggested, but this is not practiced widely (McCarthy et al., 2017). Buprenorphine is a partial mu-opioid agonist and kappa-opioid antagonist that is an acceptable substitute to pregnant women and is associated with lower risk of preterm birth, improved growth parameters at birth and less NAS, without apparent harm, when compared with methadone (Jones et al., 2010, Zedler et al., 2016). These neonatal outcomes suggest that buprenorphine may have a more favorable safety profile for the child, although long-term outcome studies are required.
Polydrug use, both illicit and prescribed, was very common among women prescribed methadone in our study. This is consistent with observations from other cohorts of methadone using pregnant women (McGlone et al., 2013b, Rosen and Johnson, 1985, van Baar, 1990). In our study population, heroin and oral benzodiazepines were used by 11 (55%) and 12 (60%) of cases respectively, so it is possible that either drug could have confounded the observed association. No other drug class (anti-depressant, anti-epileptic, stimulant) was taken by > 15% of the cases so it is unlikely that exposure to these classes of drug explained the findings. The research implication of highly prevalent polydrug use in the target population is that future studies into optimal management of OUD in pregnancy are likely to require pragmatic designs that define case definition based on prescribed substitute, with post hoc adjustment for other drug exposures if they are shown to differ between groups.
The strengths of the study are that: dMRI acquisition took place soon after birth before exposure to postnatal opioids or other pharmacological treatment for NAS; preterm birth, which is an important source of confounding for neurodevelopmental outcome was excluded; and detailed information about methadone dose and exposure to other drugs was available. Our study was limited by reliance on maternal report for drug exposures among control women; however, none of the control participants were prescribed opioids during pregnancy, and the likelihood of undisclosed heroin use or non-prescription opioids was low. A limitation of this study is that we could not evaluate causation, and therefore cannot exclude potential mediating or interacting factors such timing and dose effects of methadone and the role of other prenatal drug exposures. However, our data support pre-clinical studies, objective studies of visuo-cortical function in the newborn period and later infancy, and neurodevelopmental and behavioral studies, which strongly suggest an adverse effect of prenatal methadone upon the developing fetal brain and upon long-term childhood outcomes.
In conclusion, prenatal methadone exposure is associated with altered white matter microstructure that is apparent soon after birth. The data focus research attention on determining optimal management of pregnant women with OUD, including a pressing need to evaluate methadone dose regimens and alternative substitutes; future study designs should evaluate fetal or neonatal brain development and long-term neurocognitive outcome.
Acknowledgments
Acknowledgements
We thank the families that participated in this research; the specialist substance misuse midwives (LC and SC); and the radiographers at the Clinical Research Imaging Centre, Edinburgh, UK. We thank Thorsten Feiweier at Siemens Healthcare for collaborating with dMRI acquisitions (Works-in-Progress Package for Advanced EPI Diffusion Imaging). This work was supported by Theirworld (www.theirworld.org) and was undertaken in the MRC Centre for Reproductive Health, which is funded by MRC Centre Grant (MRC G1002033). The study was sponsored by the University of Edinburgh.
Financial disclosures
None of the authors report biomedical financial interests or potential conflicts of interest.
References
- American College of Obstetricians and Gynecologists Committee opinion no. 711: opioid use and opioid use disorder in pregnancy. Obstet. Gynecol. 2017;130:e81–e94. doi: 10.1097/AOG.0000000000002235. [DOI] [PubMed] [Google Scholar]
- Anblagan D., Pataky R., Evans M.J., Telford E.J., Serag A., Sparrow S., Piyasena C., Semple S.I., Wilkinson A.G., Bastin M.E., Boardman J.P. Association between preterm brain injury and exposure to chorioamnionitis during fetal life. Sci. Rep. 2016;6 doi: 10.1038/srep37932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjari M., Srinivasan L., Allsop J.M., Hajnal J.V., Rutherford M.A., Edwards A.D., Counsell S.J. Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. NeuroImage. 2007;35:1021–1027. doi: 10.1016/j.neuroimage.2007.01.035. [DOI] [PubMed] [Google Scholar]
- Azzopardi D., Robertson N.J., Bainbridge A., Cady E., Charles-Edwards G., Deierl A., Fagiolo G., Franks N.P., Griffiths J., Hajnal J., Juszczak E., Kapetanakis B., Linsell L., Maze M., Omar O., Strohm B., Tusor N., Edwards A.D. Moderate hypothermia within 6 h of birth plus inhaled xenon versus moderate hypothermia alone after birth asphyxia (TOBY-Xe): a proof-of-concept, open-label, randomised controlled trial. Lancet Neurol. 2016;15:145–153. doi: 10.1016/S1474-4422(15)00347-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Baar A. Development of infants of drug dependent mothers. J. Child Psychol. Psychiatry. 1990;31:911–920. doi: 10.1111/j.1469-7610.1990.tb00833.x. [DOI] [PubMed] [Google Scholar]
- Ball G., Counsell S.J., Anjari M., Merchant N., Arichi T., Doria V., Rutherford M.A., Edwards A.D., Rueckert D., Boardman J.P. An optimised tract-based spatial statistics protocol for neonates: applications to prematurity and chronic lung disease. NeuroImage. 2010;53:94–102. doi: 10.1016/j.neuroimage.2010.05.055. [DOI] [PubMed] [Google Scholar]
- Ball G., Boardman J.P., Arichi T., Merchant N., Rueckert D., Edwards A.D., Counsell S.J. Testing the sensitivity of tract-based spatial statistics to simulated treatment effects in preterm neonates. PLoS One. 2013;8:e67706. doi: 10.1371/journal.pone.0067706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesa M., Serag A., Wilkinson A.G., Anblagan D., Telford E.J., Pataky R., Sparrow S.A., Macnaught G., Semple S.I., Bastin M.E., Boardman J.P. Parcellation of the healthy neonatal brain into 107 regions using atlas propagation through intermediate time points in childhood. Front. Neurosci. 2016;10:220. doi: 10.3389/fnins.2016.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman J.P., Walley A., Ball G., Takousis P., Krishnan M.L., Hughes-Carre L., Aljabar P., Serag A., King C., Merchant N., Srinivasan L., Froguel P., Hajnal J., Rueckert D., Counsell S., Edwards A.D. Common genetic variants and risk of brain injury after preterm birth. Pediatrics. 2014;133:e1655–1663. doi: 10.1542/peds.2013-3011. [DOI] [PubMed] [Google Scholar]
- Bogen D.L., Perel J.M., Helsel J.C., Hanusa B.H., Romkes M., Nukui T., Friedman C.R., Wisner K.L. Pharmacologic evidence to support clinical decision making for peripartum methadone treatment. Psychopharmacology. 2013;225:441–451. doi: 10.1007/s00213-012-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinjikji W., El-Rida El-Masri A., Wald J.T., Lanzino G. Prevalence of developmental venous anomalies increases with age. Stroke. 2017;48:1997–1999. doi: 10.1161/STROKEAHA.116.016145. [DOI] [PubMed] [Google Scholar]
- Chen H.H., Chiang Y.C., Yuan Z.F., Kuo C.C., Lai M.D., Hung T.W., Ho I.K., Chen S.T. Buprenorphine, methadone, and morphine treatment during pregnancy: behavioral effects on the offspring in rats. Neuropsychiatr. Dis. Treat. 2015;11:609–618. doi: 10.2147/NDT.S70585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counsell S.J., Ball G., Edwards A.D. New imaging approaches to evaluate newborn brain injury and their role in predicting developmental disorders. Curr. Opin. Neurol. 2014;27:168–175. doi: 10.1097/WCO.0000000000000073. [DOI] [PubMed] [Google Scholar]
- Duerden E.G., Guo T., Dodbiba L., Chakravarty M.M., Chau V., Poskitt K.J., Synnes A., Grunau R.E., Miller S.P. Midazolam dose correlates with abnormal hippocampal growth and neurodevelopmental outcome in preterm infants. Ann. Neurol. 2016;79:548–559. doi: 10.1002/ana.24601. [DOI] [PubMed] [Google Scholar]
- Frank D.A., McCarten K.M., Robson C.D., Mirochnick M., Cabral H., Park H., Zuckerman B. Level of in utero cocaine exposure and neonatal ultrasound findings. Pediatrics. 1999;104:1101–1105. doi: 10.1542/peds.104.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans S.L., Jeremy R.J. Postneonatal mental and motor development of infants exposed in utero to opioid drugs. Infant Mental Health Journal. 2001;22:15. [Google Scholar]
- Hunt R.W., Tzioumi D., Collins E., Jeffery H.E. Adverse neurodevelopmental outcome of infants exposed to opiate in-utero. Early Hum. Dev. 2008;84:29–35. doi: 10.1016/j.earlhumdev.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Jansson L.M., Dipietro J.A., Velez M., Elko A., Knauer H., Kivlighan K.T. Maternal methadone dosing schedule and fetal neurobehaviour. J. Matern. Fetal Neonatal Med. 2009;22:29–35. doi: 10.1080/14767050802452291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H.E., Kaltenbach K., Heil S.H., Stine S.M., Coyle M.G., Arria A.M., O'Grady K.E., Selby P., Martin P.R., Fischer G. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N. Engl. J. Med. 2010;363:2320–2331. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konijnenberg C., Melinder A. Visual selective attention is impaired in children prenatally exposed to opioid agonist medication. Eur. Addict. Res. 2015;21:63–70. doi: 10.1159/000366018. [DOI] [PubMed] [Google Scholar]
- Leuchter R.H., Gui L., Poncet A., Hagmann C., Lodygensky G.A., Martin E., Koller B., Darque A., Bucher H.U., Huppi P.S. Association between early administration of high-dose erythropoietin in preterm infants and brain MRI abnormality at term-equivalent age. JAMA. 2014;312:817–824. doi: 10.1001/jama.2014.9645. [DOI] [PubMed] [Google Scholar]
- Mactier H., Shipton D., Dryden C., Tappin D.M. Reduced fetal growth in methadone-maintained pregnancies is not fully explained by smoking or socio-economic deprivation. Addiction. 2014;109:482–488. doi: 10.1111/add.12400. [DOI] [PubMed] [Google Scholar]
- McCarthy J.J., Leamon M.H., Willits N.H., Salo R. The effect of methadone dose regimen on neonatal abstinence syndrome. J. Addict. Med. 2015;9:105–110. doi: 10.1097/ADM.0000000000000099. [DOI] [PubMed] [Google Scholar]
- McCarthy J.J., Leamon M.H., Finnegan L.P., Fassbender C. Opioid dependence and pregnancy: minimizing stress on the fetal brain. Am. J. Obstet. Gynecol. 2017;216:226–231. doi: 10.1016/j.ajog.2016.10.003. [DOI] [PubMed] [Google Scholar]
- McGlone L., Mactier H. Infants of opioid-dependent mothers: neurodevelopment at six months. Early Hum. Dev. 2015;91:19–21. doi: 10.1016/j.earlhumdev.2014.10.006. [DOI] [PubMed] [Google Scholar]
- McGlone L., Mactier H., Hamilton R., Bradnam M.S., Boulton R., Borland W., Hepburn M., McCulloch D.L. Visual evoked potentials in infants exposed to methadone in utero. Arch. Dis. Child. 2008;93:784–786. doi: 10.1136/adc.2007.132985. [DOI] [PubMed] [Google Scholar]
- McGlone L., Hamilton R., McCulloch D.L., Boulton R., Bradnam M.S., Weaver L.T., Mactier H. Neonatal visual evoked potentials in infants born to mothers prescribed methadone. Pediatrics. 2013;131:e857–863. doi: 10.1542/peds.2012-2113. [DOI] [PubMed] [Google Scholar]
- McGlone L., Mactier H., Hassan H., Cooper G. In utero drug and alcohol exposure in infants born to mothers prescribed maintenance methadone. Arch Dis Child Fetal Neonatal Ed. 2013;98:F542–544. doi: 10.1136/archdischild-2013-304158. [DOI] [PubMed] [Google Scholar]
- McGlone L., Hamilton R., McCulloch D.L., MacKinnon J.R., Bradnam M., Mactier H. Visual outcome in infants born to drug-misusing mothers prescribed methadone in pregnancy. Br. J. Ophthalmol. 2014;98:238–245. doi: 10.1136/bjophthalmol-2013-303967. [DOI] [PubMed] [Google Scholar]
- O'Gorman R.L., Bucher H.U., Held U., Koller B.M., Huppi P.S., Hagmann C.F. Tract-based spatial statistics to assess the neuroprotective effect of early erythropoietin on white matter development in preterm infants. Brain. 2015;138:388–397. doi: 10.1093/brain/awu363. [DOI] [PubMed] [Google Scholar]
- Porter E.J., Counsell S.J., Edwards A.D., Allsop J., Azzopardi D. Tract-based spatial statistics of magnetic resonance images to assess disease and treatment effects in perinatal asphyxial encephalopathy. Pediatr. Res. 2010;68:205–209. doi: 10.1203/PDR.0b013e3181e9f1ba. [DOI] [PubMed] [Google Scholar]
- Robinson S.E., Guo H., Maher J.R., McDowell K.P., Kunko P.M. Postnatal methadone exposure doe not prevent prenatal methadone-induced changes in striatal cholinergic neurons. Brain Res. Dev. Brain Res. 1996;95:118–121. doi: 10.1016/0165-3806(96)00045-4. [DOI] [PubMed] [Google Scholar]
- Rosen T.S., Johnson H.L. Long-term effects of prenatal methadone maintenance. NIDA Res. Monogr. 1985;59:73–83. [PubMed] [Google Scholar]
- Salzwedel A.P., Grewen K.M., Vachet C., Gerig G., Lin W., Gao W. Prenatal drug exposure affects neonatal brain functional connectivity. J. Neurosci. 2015;35:5860–5869. doi: 10.1523/JNEUROSCI.4333-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzwedel A.P., Grewen K.M., Goldman B.D., Gao W. Thalamocortical functional connectivity and behavioral disruptions in neonates with prenatal cocaine exposure. Neurotoxicol. Teratol. 2016;56:16–25. doi: 10.1016/j.ntt.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- United Nations Office of Drugs and Crime . United Nations; 2017. World Drug Report 2017. United Nations publication, Sales No. E.17.XI.6. [Google Scholar]
- Vestal-Laborde A.A., Eschenroeder A.C., Bigbee J.W., Robinson S.E., Sato-Bigbee C. The opioid system and brain development: effects of methadone on the oligodendrocyte lineage and the early stages of myelination. Dev. Neurosci. 2014;36:409–421. doi: 10.1159/000365074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar J., Cheikh Ismail L., Victora C.G., Ohuma E.O., Bertino E., Altman D.G., Lambert A., Papageorghiou A.T., Carvalho M., Jaffer Y.A., Gravett M.G., Purwar M., Frederick I.O., Noble A.J., Pang R., Barros F.C., Chumlea C., Bhutta Z.A., Kennedy S.H. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384:857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- Walhovd K.B., Watts R., Amlien I., Woodward L.J. Neural tract development of infants born to methadone-maintained mothers. Pediatr. Neurol. 2012;47:1–6. doi: 10.1016/j.pediatrneurol.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Whitham J.N., Spurrier N.J., Sawyer M.G., Baghurst P.A., Taplin J.E., White J.M., Gordon A.L. The effects of prenatal exposure to buprenorphine or methadone on infant visual evoked potentials. Neurotoxicol. Teratol. 2010;32:280–288. doi: 10.1016/j.ntt.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Wilson G.S. Clinical studies of infants and children exposed prenatally to heroin. Ann. N. Y. Acad. Sci. 1989;562:183–194. doi: 10.1111/j.1749-6632.1989.tb21017.x. [DOI] [PubMed] [Google Scholar]
- Wilson G.S., Desmond M.M., Wait R.B. Follow-up of methadone-treated and untreated narcotic-dependent women and their infants: health, developmental, and social implications. J. Pediatr. 1981;98:716–722. doi: 10.1016/s0022-3476(81)80830-x. [DOI] [PubMed] [Google Scholar]
- Wittmann B.K., Segal S. A comparison of the effects of single- and split-dose methadone administration on the fetus: ultrasound evaluation. Int. J. Addict. 1991;26:213–218. doi: 10.3109/10826089109053183. [DOI] [PubMed] [Google Scholar]
- Wong C.S., Lee Y.J., Chiang Y.C., Fan L.W., Ho I.K., Tien L.T. Effect of prenatal methadone on reinstated behavioral sensitization induced by methamphetamine in adolescent rats. Behav. Brain Res. 2014;258:160–165. doi: 10.1016/j.bbr.2013.10.027. [DOI] [PubMed] [Google Scholar]
- Woodward L.J., Anderson P.J., Austin N.C., Howard K., Inder T.E. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N. Engl. J. Med. 2006;355:685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- Zedler B.K., Mann A.L., Kim M.M., Amick H.R., Joyce A.R., Murrelle E.L., Jones H.E. Buprenorphine compared with methadone to treat pregnant women with opioid use disorder: a systematic review and meta-analysis of safety in the mother, fetus and child. Addiction. 2016;111:2115–2128. doi: 10.1111/add.13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelson C., Lee S.J., Casalino M. Neonatal narcotic addiction. Comparative effects of maternal intake of heroin and methadone. N. Engl. J. Med. 1973;289:1216–1220. doi: 10.1056/NEJM197312062892303. [DOI] [PubMed] [Google Scholar]