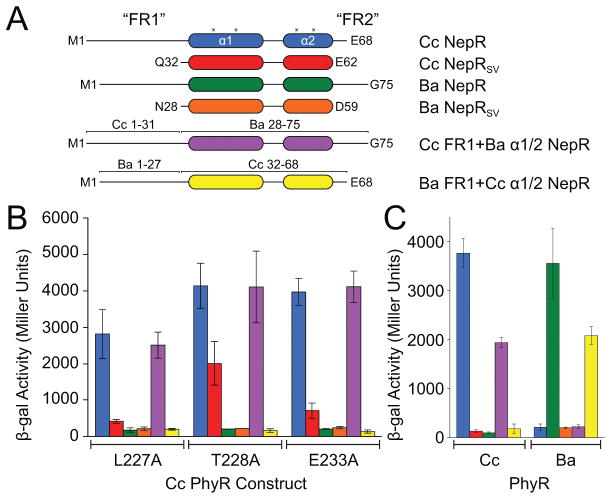
Figure 6. Intrinsically disordered NepR N-terminal sequences confer PhyR-NepR interaction specificity.
(A) C. crescentus (Cc) and B. abortus (Ba) NepR constructs used in bacterial two-hybrid (BTH) interaction assays: SV, a NepR construct lacking both FR1 and FR2; Cc FR1+Ba 1/2 NepR is a chimeric construct containing Cc NepR residues 1–31 fused to Ba NepR residues 28–75; Ba FR1+Cc 1/2 NepR is a chimeric construct containing Ba NepR residues 1–27 fused to Cc NepR residues 32–68; asterisk marks location of residues previously identified as required for PhyR-NepR binding (Campagne et al., 2012). (B) BTH interaction assay between NepR constructs (labelled by color from panel A) and three constitutively active PhyR mutants (labelled on axis). (C) Interaction of wild-type and chimeric NepR constructs (by color from panel A) with wild-type C. crescentus (Cc) or B. abortus (Ba) PhyR. Protein stability controls are presented in Figure S7. Error bars represent standard deviation.