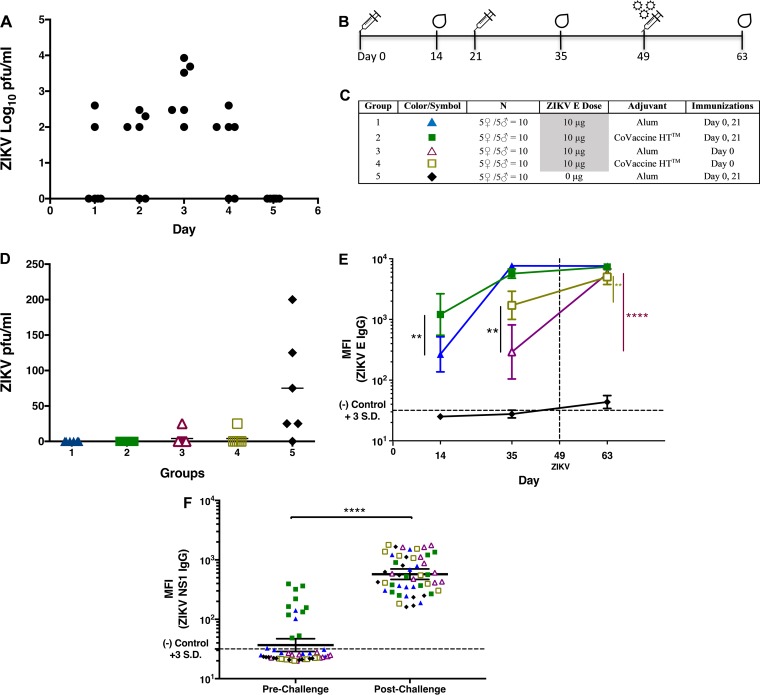
FIG 4 .
Immunization of mice with recombinant ZIKV E protein vaccine inhibits viral replication postchallenge. (A) Kinetics of viremia in naive BALB/c mice challenged intravenously (i.v.) with 100 PFU of ZIKV Puerto Rican strain PRVABC59. Six animals of both sexes were euthanized daily after infection, and total serum was collected from each animal. Viremia was measured for each serum sample using a standard plaque assay on Vero cells. The limit of detection was 101.7 PFU/ml. (B) Vaccination schedule for the BALB/c mouse challenge study. (C) Experimental groups. Alum, 2% Alhydrogel adjuvant. (D) Viremia in vaccinated and control mice on day 3 after infection. Mice vaccinated once or twice were challenged as described for panel A. Viremia on day 3 postchallenge was measured as described for panel A. The limit of detection was 25 PFU/ml. (E) GMTs expressed as the MFIs (with 95% CI) of ZIKV E-specific IgG titers observed in serum samples collected 2 weeks after immunization and 2 weeks after ZIKV challenge measured by MIA. (F) Anti-NS1 antibody titers in vaccinated and control mice pre- and postchallenge. Antibody titers were measured by MIA on individual serum samples at a 1:100 dilution. **, P < 0.01; ****, P < 0.0001.