Abstract
Rapid and sensitive reversed phase high performance liquid chromatography (RP-HPLC) and ultra performance liquid chromatography (RP-UPLC) method with UV detection has been developed and validated for quantification of parathyroid hormone (PTH) in presence of meta-cresol as a stabilizer in a pharmaceutical formulation. Chromatography was performed with mobile phase containing 0.1% Trifluoroacetic acid (TFA) in MilliQ water and 0.1% TFA in acetonitrile with gradient program and flow rate at 0.3 mL/min for HPLC and 0.4 mL/min for UPLC. Quantification was accomplished with internal reference standard (qualified against innovator product and National Institute for Biological Standards and Control (NIBSC) standard). The methods were validated for linearity (correlation coefficient=0.99), range, accuracy, precision and robustness. Robustness was confirmed by considering three factors; mobile phase composition, column temperature and flow rate/age of mobile phase.
Intermediate precision was confirmed on different equipments, different columns and on different days. The relative standard deviation (RSD) (<2% for RP-HPLC and <1% for UPLC, n=30) indicated a good precision. Retention time was found about 17 min and 2 min by HPLC and UPLC methods, respectively. Both methods are simple, highly sensitive, precise and accurate and have the potential of being useful for routine quality control.
Keywords: Parathyroid hormone, Reversed phase high performance liquid chromatography, Ultra performance liquid chromatography, Validation, Meta-cresol
1. Introduction
Parathyroid hormone (PTH) is secreted by the parathyroid glands as a polypeptide containing 84 amino acids. It acts to increase the concentration of calcium (Ca2+) in the blood. A smaller fragment of the full length hormone PTH (1-34) crystallizes as a slightly bent, long helical dimer. Analysis reveals that the extended helical conformation of PTH (1-34) is the likely bioactive conformation [1]. Human parathyroid hormone, a peptide of 84 amino acid residues [2] secreted from parathyroid gland, is the principle homeostatic regulator of the level of blood calcium through its actions on kidney and bone [3]. Teriparatide (recombinant DNA origin) injection [recombinant human PTH (1-34)] is a bone-forming agent for the treatment of osteoporosis. In the Fracture Prevention Trial (FPT), daily self-injections of teriparatide (20 and 40 μg) reduced the risk of new vertebral and non-vertebral fractures by 65% and 53%, respectively, in postmenopausal women with advanced osteoporosis [4]. Once-daily injection of PTH induced pronounced increase in biochemical markers of bone turnover [5], [6], [7], [8], [9], [10].
Immunoassay is a common technique for measurement of PTH in plasma. The measurement of PTH and its metabolites has been problematic due to the diversity of the circulating PTH metabolites, differences in the pharmacokinetic profiles of PTH and its metabolites and significant differences in specificity and sensitivity of PTH radioimmunoassay [11], [12], [13]. Methionine oxidation in PTH was studied by Nabuchi et al. by using RP-HPLC [14]. WHO International collaborative study of the proposed 1st international standard for recombinant human PTH (1-84) was done by RP-HPLC method in different laboratories [15]. Liquid chromatographic studies on separation of ten PTH amino acids were carried out using normal phase untreated silica gel plate, C-18 RP precoated plates and RP-HPLC by Bhushan and Agarwal [16]. Separation, characterization and biological activity of PTH oxidized at methionine 8 and methionine 18 were studied by Frelinger and Zulls [17]. PTH was oxidized with hydrogen peroxide and the biological activity of oxidation products was studied by Nabuchi et al. [18].
The recombinant human (rHu) PTH (1-34) formulation contains meta-cresol as antimicrobial preservative, which may interfere with OD280 UV detector of HPLC as well as with colorimetric assays. An RP-HPLC/UPLC method, which can specifically measure the protein component of Active Pharmaceutical Ingredient (API) with separation of meta-cresol from protein peaks, will be suitable for quantitation of the active substance in presence of meta-cresol. The objective of the study was to develop methods, using RP-HPLC and UPLC techniques to enable quantification of PTH in medicinal formulations containing meta-cresol.
This paper reports rapid and sensitive methods with UV detection, which are useful for routine quality control of PTH in pharmaceutical formulations. Both the methods are validated by parameters such as linearity, accuracy, precision and robustness. To the best of our knowledge, no UPLC method has been reported.
2. Experimental
2.1. Materials, reagents and chemicals
HPLC grade acetonitrile and methanol were purchased from Merck, tri-fluoro-acetic acid was purchased from Sigma Aldrich. Ultra pure water was obtained using Milli-Q® UF-Plus (Millipore) system, meta-cresol was obtained from J.T. Baker/Hedinger. Reference medicinal product (Innovator drug product) having a concentration of 250 μg/mL and PTH internal reference standard (IRS) were used for preparation of standards in all experiments. Formulated PTH was used as samples. All other chemicals such as mannitol, sodium acetate and glacial acetic were of the highest purity available.
2.2. Preparation of standard, mobile phase and dilution buffer
Diluted PTH standard was prepared using 400 μg/mL of PTH IRS in mobile phase A. Mobile phase ‘A’ consisted of 0.1% (v/v) TFA in MilliQ water and mobile phase ‘B’ consisted of 0.1% (v/v) TFA in acetonitrile. Dilution buffer containing 3 mg/mL meta-cresol, 45.4 mg/mL mannitol, 0.1 mg/mL sodium acetate and 0.41 mg/mL glacial acetic acid in “MilliQ water” was prepared and used so as to have a matrix similar to PTH formulation. Oxidized form of PTH was prepared by adding 4.0 μL of diluted 0.25% H2O2 to 62.6 μL of PTH drug substance (0.4 mg/mL), mixed well, incubated for 40 min at room temperature and then quenched with 37.4 μL of 50 mg/mL methionine. All dilutions were made using calibrated digital micro-pipettes.
2.3. Chromatographic condition
Agilent LC system (1100 and 1200 series) equipped with an injection valve (quaternary), 210 UV detector and Chemstation software was used for HPLC method. A reversed-phase C18 column (2.1 mm ID×100 mm L, porosity 300 Å, particle size 3 μm) with guard column (reversed-phase C18 column of 2.1 mm ID×12.5 mm L, porosity 300 Å, particle size 5 μm) was used for separation. To get the optimum results, mobile phase with a flow rate of 0.3 mL/min was used and column temperature was maintained at 60 °C. The gradient program for mobile phase was optimized using a timed gradient program T (min)/mobile phase A (%): 0/80, 6/80, 26.1/45, 28/0, 31/0, 31.5/80, and 40/80.
Waters LC system (ACQUITY) equipped with an injection valve (binary), 215UV detector and Empower software was used for RP-UPLC method. Reversed-phase C8 column (2.1 mm ID×12.5 mm L, porosity 300 Å, particle size 5 μm) was used for separation. To get the optimum results, mobile phase flow rate was kept constant at 0.4 mL/min, column temperature at 60 °C. The gradient program for mobile phase was optimized using a timed gradient program T (min)/mobile phase A (%): 0/80, 1.2/80, 4.8/0, 5/80, and 6/80.
2.4. Validation of chromatographic methods
Method validation is the process used to confirm that the analytical procedure employed for a specific test is suitable for its intended use [19], [20]. The optimized chromatographic methods were validated according to the procedures described in International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) guidelines Q2 (R1) in terms of accuracy, precision, specificity, linearity and range, robustness, etc. [21].
3. Results and discussion
3.1. RP-HPLC method
3.1.1. Method development
Initially, the gradient HPLC conditions were optimized for determination of PTH in presence of meta-cresol. The chromatographic separation was achieved by applying chromatographic conditions described in Section 2.3.
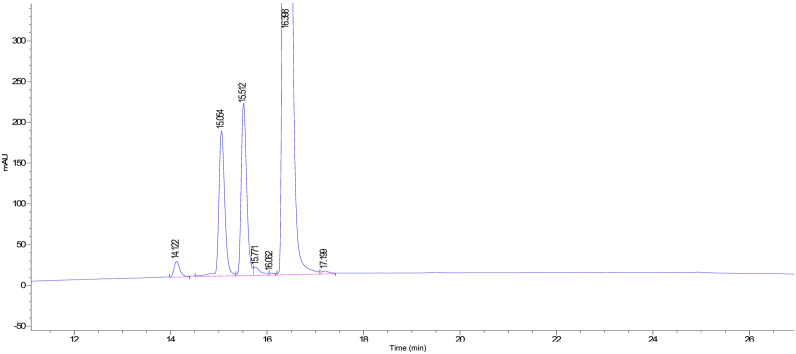
The applied chromatographic conditions permitted a good separation of meta-cresol and PTH at different concentrations of PTH. No interference of other excipients or oxidized impurities was observed (Figure 1, Figure 2).
Figure 1.
Overlapped HPLC chromatograms of (A) mobile phase (as blank), (B) formulation buffer and (C) PTH drug product. Mobile phase, formulation buffer and PTH drug product were injected into HPLC separately. (A) Mobile phase—containing 0.1% trifluoroacetic acid (TFA) in MilliQ water and 0.1% TFA in acetonitrite. (B) Formulation buffer (without meta-cresol)—containing 45.5 mg/mL mannitol, 0.3 mg/mL sodium acetate and 0.41 mg/mL glacial acetic acid in MilliQ water. (C) PTH drug product—containing 0.250 mg/mL of PTH and mg/mL ‘meta-cresol’.
Figure 2.
HPLC chromatogram of PTH oxidized form. Oxidized form of PTH was prepared by adding 4.0 μL of diluted 0.25% H2O2 to 62.6 mL of PTH drug substance (0.4 mg/mL), mixed well, incubated for 40 min at room temperature and then quenched with 37.4 μL of 50 mg/mL methionine.
The capacity factor (k′) of the first peak (meta-cresol) and second peak (PTH) was 1.60 and 11.5, respectively; while the resolution factor was 6.88. The asymmetry of the peak for meta-cresol and PTH was found to be 0.26 and 1.06, respectively; while the tailing factor parameter for meta-cresol and PTH was found to be 3.62 and 1.36, respectively.
Based on the studied parameters, it was concluded that the developed method is optimum. PTH, oxidized impurities and meta-cresol peaks were well resolved and the tailing factor was within limits.
3.1.2. Method validation
3.1.2.1. Specificity
To evaluate possible interfering peaks, PTH standard (250 μg/mL) in mobile phase (as positive control), API, drug product (to verify the separation of interested protein from other components) and oxidized API (to confirm the separation of oxidized forms of protein from the interested protein) were injected into HPLC and no interference was observed (Fig. 2).
3.1.2.2. Linearity and range
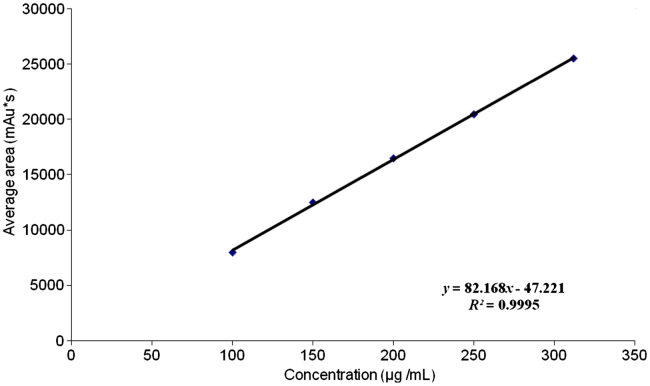
PTH IRS was used for preparation of different concentrations ranging from 100 to 312 μg/mL. Linearity curve was plotted for of peak area responses versus concentration of PTH, which is shown in Fig. 3. The correlation coefficient, slope, Y-intercept, regression equation of the calibration curve were determined and the results are shown in Table 1. The percent RSD was found to be less than 2.0% while the percent recovery was found to be in the range of 97–103%.
Figure 3.
Linearity curve (HPLC) for PTH. PTH drug substance (400 μg/mL) was diluted with mobile phase A for preparation of different concentrations ranging from 100 to 312 μg/mL and injected separately.
Table 1.
Results of regression equation/correlation coefficient and both methods comparative data.
| Statistical parameter | RP-HPLC | UPLC |
|---|---|---|
| Linearity and range (concentration, μg/mL) | 100–300 | 50–300 |
| Regression equation | y=82.168×−47.221 | y=12203.8226×−94815.0889 |
| Correlation coefficient (R2) | 0.999 | 0.999 |
| Total analysis time (min) | 40 | 6 |
| Retention time (min) | ||
| For meta-cresol | 3.5 | 0.3 |
| For PTH | 16.4 | 1.9 |
| Flow rate (mL/min) | 0.3 | 0.4 |
| Column | C18 | C8 |
| (2.1 mm×100 mm) | (2.1 mm×12.5 mm) | |
| 3 μm, 300 Å | 5 μm, 300 Å | |
| Column condition | C18, 210 UV | C8, 215 UV |
| Method | Gradient | Gradient |
| Sample size | 20 μL | 4 μL |
| Specificity | No interference | No interference |
| Accuracy | Recovery between 95% and 105% | Recovery between 95% and 105% |
| Precision | RSD <3.0% | RSD <1.0% |
| Robustness | Yes | Yes |
3.1.2.3. Accuracy
Accuracy was studied by spiking PTH in the range of 200, 250 and 312 μg/mL in the mobile phase. The percent recovery was found to be in the range of 95–105%. The percent RSD was found to be less than 2.0%.
3.1.2.4. Precision
Precision was evaluated on intra-day (Repeatability) and inter-day (intermediate precision) variation, and different columns. Intra-day study was determined by performing six independent preparation of the standard preparation of drug substance (250 μg/mL) and drug product (250 μg/mL). The percent RSD of main peak area was found to be less than 0.5%. Inter-day precision was determined by performing five different conditions along with the five replicates for each condition, which is equivalent to n=25 (5×5). The percent RSD of the main peak area was found to be less than 0.5% within each set and less than 2.0% between different sets. The percent recovery was found to be between 95.0% and 105.0% and the maximum variation between sets was found to be less than 5.0%.
3.1.2.5. Robustness
The robustness is a measure of its capacity to remain unaffected by small, but deliberate variations in method parameters and provides an indication of its robustness during normal usage. Robustness was tested using three variables: flow rate, column temperature and mobile phase composition.
3.1.2.6. Flow rate
Experiments were conducted using system suitability samples with flow rate variation of +10% from the set flow rate (0.3 mL/min). The percent RSD was found to be less than 2%, with no variation and +0.1 min difference in retention time but during lower side of flow rate, we found higher percentage of recovery (i.e. about 111%) whereas during higher side of flow rate, we found lower percentage of recovery (i.e. about 93%). Based on recovery, it was concluded that flow rate is critical parameter.
3.1.2.7. Column temperature effect
Experiments were conducted using system suitability samples with column temperature variation of +5 °C from the set temperature (60 °C). The percent RSD was found to be less than 2%, with no variation and +0.1 min difference in retention time. The percent recovery was found to be within acceptable limits (95–105%) and hence column temperature was not considered to be critical parameter.
3.1.2.8. Mobile phase composition
Experiments were conducted using system suitability samples with mobile phase composition variation of ±20% from the set percentage of TFA (0.1%). Results for triplicate injections (% variation) between unaltered/initial condition and altered condition for PTH sample were found to vary less than 2.0% without variation in retention time and hence mobile phase composition was not considered to be critical parameter.
3.2. UPLC method
3.2.1. Method development
The basic chromatographic conditions like stationary phase, solvents and UV detector, employed in HPLC were taken into account while developing new UPLC method. The stationary phase C8 was chosen in order to have similar polarity as that used in HPLC. The injection volume was scaled down by about 5 fold as used in HPLC. To get the optimum results, mobile phase flow rate was kept constant at 0.4 mL/min and column temperature was maintained at 60 °C. The chromatographic separation was achieved as described in Section 2.3.
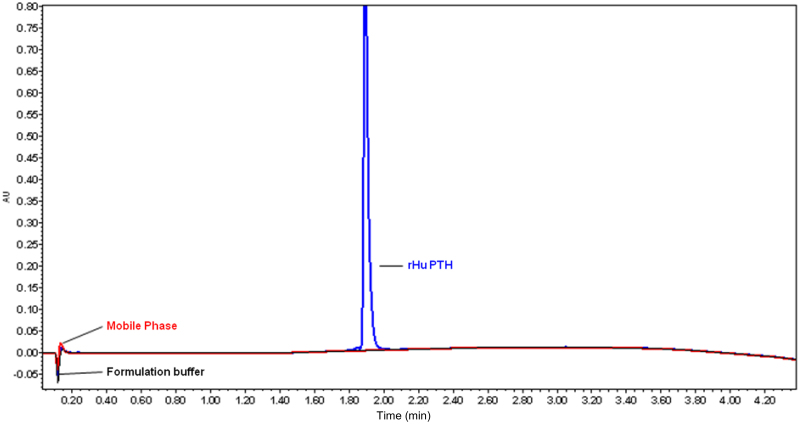
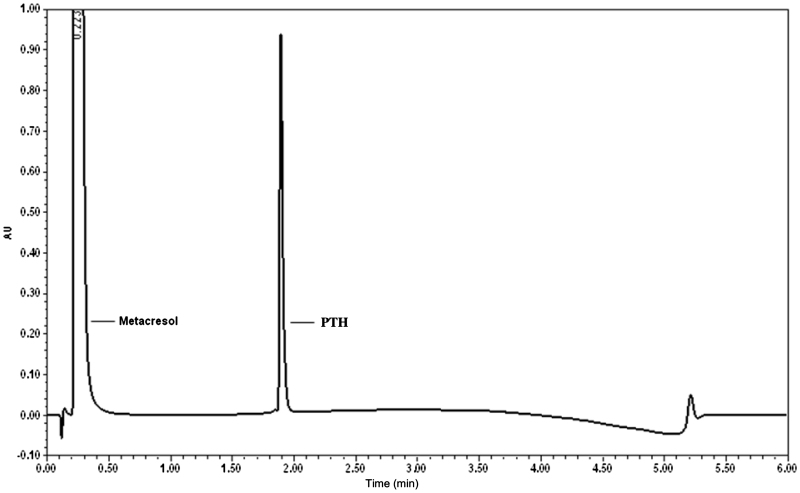
The applied chromatographic conditions permitted a good separation of meta-cresol and PTH at different concentrations of PTH. No interference of other excipients or other oxidized impurities was observed during the analysis (Figure 4, Figure 5, Figure 6).
Figure 4.
Overlapped UPLC chromatograms of (A) mobile phase, (B) formulation buffer and (C) PTH drug substance (without meta-cresol). Mobile phase, formulation buffer and PTH were injected into HPLC separately. (A) Mobile phase—containing 0.1% trifluoroacetic acid (TFA) in MilliQ water and 0.1% TFA in acetonitrite. (B) Formulation buffer (without meta-cresol)—containing 45.5 mg/mL mannitol, 0.1 mg/mL sodium acetate and 0.41 mg/mL glacial acetic acid in MilliQ water. (C) PTH drug substance—containing 0.250 mg/mL of PTH in MilliQ water.
Figure 5.
UPLC chromatograms of meta cresol and PTH drug product (with meta-cresol). PTH drug product—containing 0.250 mg/mL of PTH and 3 mg/mL ‘meta-cresol’.
Figure 6.
UPLC chromatogram of PTH oxidized form. Oxidized form of PTH was prepared by adding 4.0 μL of diluted 0.25% H2O2 to 62.6 μL of PTH drug substance (0.4 mg/mL), mixed well, incubated for 40 min at room temperature and then quenched with 37.4 μL of 50 mg/mL methionine.
The capacity factor (k′) of the main peak (PTH) was 11.60; while tailing factor was found to be 1.24. It can be thus concluded that PTH, oxidized forms of PTH and meta-cresol peaks were well resolved in the developed method and the tailing factor was within limits.
3.2.2. Method validation
3.2.2.1. Specificity
To evaluate possible interfering peaks, PTH standard (150 μg/mL) in mobile phase (as positive control), API, drug product (to verify the separation of interested protein from other components), oxidized API and oxidized drug product (to confirm the separation of oxidized forms of protein from the interested protein) were injected into UPLC and no interference was observed (Fig. 6).
3.2.2.2. Linearity and range
PTH RS and samples were chromatographed using the set chromatographic conditions. Linearity curves were plotted for 50–300 μg/mL of PTH (Fig. 7). The linearity of peak area responses versus concentration for PTH was studied and correlation coefficient, slopes and Y-intercepts and regression equation were determined and the results are shown in Table 1. The correlation coefficient was found to be 0.999. The percent RSD was found to be less than 2.0% while the percent recovery was found to be in the range of 98–105%.
Figure 7.
Linearity curve (UPLC) for PTH. PTH drug substance (400 μg/mL) was diluted with mobile phase ‘A’ for preparation of different concentrations ranging from 50 to 300 μg/mL, which were injected separately.
3.2.2.3. Accuracy
Accuracy was studied using six different solutions, containing 50, 100, 150, 200, 250 and 300 μg/mL of PTH. Three replicates of each solution were spiked in the mobile phase. (n=6×3=18). The percent recovery was found to be in the range of 95–105%. The percent RSD was found to be less than 2.0%.
3.2.2.4. Precision
Precision was evaluated on intra-day (Repeatability) and inter-day (intermediate precision) e.g. variation between different equipment, and same makes but different lots of UPLC columns were studied. Intra-day study was determined by performing six independent preparation of the standard preparation of drug substance (150 μg/mL). The percent RSD of main peak area was found to be less than 0.5%. Inter-day precision was determined by performing five different conditions along with the six replicates for each condition, which is equivalent to n=30 (5×6). The percent RSD of the main peak area was found to be less than 0.5% within each set and less than 3.0% between different sets. The percent recovery was found to be between 95.0% and 105.0% and the maximum variation between sets was found to be less than 5.0%.
The intra-day (repeatability) (n=6) was assessed with six independent sample preparations for 150 μg/mL of PTH. Inter-day (intermediate precision) was determined by performing five different types of condition along with the six replicates, which is equivalent to n=30 (5×6) and percent RSD for the main peak area within set and between different sets was found to be less than 1.0%. The percent recovery of PTH standard was found to be between 95% and 105% within set and maximum variation between sets was found to be 5.0%.
3.2.2.5. Robustness
The robustness is a measure of its capacity to remain unaffected by small, but deliberate variations in method parameters and provides an indication of its robustness during normal usage. Robustness was tested using three variables, age effect of mobile phase and test samples, column temperature and mobile phase composition.
3.2.2.6. Age effect of mobile phase and test samples held for seven days
Freshly prepared samples for system suitability (150 μg/mL of PTH) and those prepared seven days ago were analyzed using both freshly prepared and seven days, old mobile phase. There was not much variation in the results, with percent variation from initial day to 7 day being about 5% and percent RSD being less than 1.0%. There was no difference in the retention time and percent recovery was found to be in between 95% and 105%, indicating that age of the mobile phase was not a critical parameter.
3.2.2.7. Column temperature effect
Experiments were conducted using system suitability samples with column temperature variation of +5 °C from the set temperature (60 °C). The percent RSD was found to be less than 2%, with variation of +0.1 min in the retention time. The percent recovery was found to be within acceptable limits (95–105%) suggesting that the variation in the results was within acceptable limits for all the parameters under study and indicating that column temperature was not a critical parameter.
3.2.2.8. Mobile phase composition
Experiments were conducted using system suitability samples with mobile phase composition variation of ±20% from the set percentage of TFA (0.1%). Results for triplicate injections (% variation) between initial condition and altered condition for PTH sample were found to vary less than 2.0% and there was no variation observed in the retention time, suggesting that the mobile phase composition was not a critical parameter.
3.3. Comparative study of HPLC and UPLC performance
The performance parameters of both systems are shown in Table 1. The runtime of UPLC was reduced by 7-fold to that of HPLC. The retention behaviors of meta-cresol, PTH, and oxidized impurities were similar in HPLC and UPLC columns. As expected, the UPLC method showed higher efficiency of analysis than HPLC method.
4. Conclusion
Both RP-HPLC and RP-UPLC methods were demonstrated to be validated for quantifying PTH, respectively, in presence of other excipients and oxidized impurities of PTH. The HPLC and UPLC methods were validated showing satisfactory data for all the parameters tested. The UPLC method was found to be capable of giving faster analysis with good resolution, accuracy and precision than that achieved with conventional HPLC method. Both the chromatographic methods described here were found to be reliable for quantifying PTH. Since these methods are rapid and simple, they may be successfully applied to quality control analyses of finished (formulated) product in presence of meta-cresol.
References
- 1.Jin L., Briggs S.L., Chandrasekhar S. Crystal structure of human parathyroid hormone 1-34 at 0.9-A resolution. J. Biol. Chem. 2000;275(35):27238–27244. doi: 10.1074/jbc.M001134200. [DOI] [PubMed] [Google Scholar]
- 2.Hendy G.N., Kronengerg H.M., Potts J.T. Nucleotide sequence of cloned cDNAs encoding human preproparathyroid hormone. Proc. Natl. Acad. Sci. USA. 1981;78(12):7365–7369. doi: 10.1073/pnas.78.12.7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potts J.T., Jr., Kronengerg H.M., Rosenblatt M. Parathyroid hormone: chemistry, biosynthesis, and mode of action. Adv. Protein Chem. 1982;35:323–396. doi: 10.1016/s0065-3233(08)60471-4. [DOI] [PubMed] [Google Scholar]
- 4.Neer R.M., Arnaud C.D., Zanchetta J.R. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 5.Reeve J., Bradbeer J.N., Arlot M. hPTH 1-34 treatment of osteoporosis with added hormone replacement therapy: biochemical, kinetic and histological responses. Osteoporos. Int. 1991;1:162–170. doi: 10.1007/BF01625448. [DOI] [PubMed] [Google Scholar]
- 6.Hodsman A.B., Fraher L.J., Ostbye T. An evaluation of several biochemical markers for bone formation and resorption in a protocol utilizing cyclical parathyroid hormone and calcitonin therapy for osteoporosis. J. Clin. Invest. 1993;91:1138–1148. doi: 10.1172/JCI116273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodsman A.B., Fraher L.J., Watson P.H. A randomized controlled trial to compare the efficacy of cyclical parathyroid hormone versus cyclical parathyroid hormone and sequential calcitonin to improve bone mass in postmenopausal women with osteoporosis. J. Clin. Endocrinol. Metab. 1997;82:620–628. doi: 10.1210/jcem.82.2.3762. [DOI] [PubMed] [Google Scholar]
- 8.Lindsay R., Nieves J., Formica C. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet. 1997;350:550–555. doi: 10.1016/S0140-6736(97)02342-8. [DOI] [PubMed] [Google Scholar]
- 9.Lane N.E., Sanchez S., Modin G.W. Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. Results of a randomized controlled clinical trial. J. Clin. Invest. 1998;102:1627–1633. doi: 10.1172/JCI3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lane N.E., Sanchez S., Genant H.K. Short-term increases in bone turnover markers predict parathyroid hormone-induced spinal bone mineral density gains in postmenopausal women with glucocorticoid-induced osteoporosis. Osteoporos. Int. 2000;11:434–442. doi: 10.1007/s001980070111. [DOI] [PubMed] [Google Scholar]
- 11.Armitage E.K. Parathyrin (parathyroid hormone): metabolism and methods for assay. Clin. Chem. 1986;32:418–424. [PubMed] [Google Scholar]
- 12.Endres D.B., Villanueva R., Singer F.R. Measurement of parathyroid hormone. Endocrinol. Metab. Clin. North Am. 1989;18:611–629. [PubMed] [Google Scholar]
- 13.Segre G.V., Potts J.T., Jr. vol. 2. W.B. Saunders company; PA: 1989. (Differential Diagnosis of Hypercalcemia. Methods and Clinical Applications of Parathyroid Assays, Endocrinology). pp. 984–1001. [Google Scholar]
- 14.Nabuchi Y., Fujiwara E., Kuboniwa H. Kinetic study of methionine oxidation in human parathyroid hormone. Anal. Chim. Acta. 1998;365:301–307. [Google Scholar]
- 15.C. Burns, M. Moore, C. Sturgeon, et al., WHO International Collaborative Study of the proposed 1st International Standard for Parathyroid Hormone 1-84, Human, Recombinant, 2009, WHO/BS/09.2115.
- 16.Bhushan R., Agarwal R. Liquid chromatographic separation of some PTH-amino acids. Biomed. Chromatogr. 1998;12:322–325. doi: 10.1002/(SICI)1099-0801(199811/12)12:6<322::AID-BMC754>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Frelinger A.L., Zulls J.E. Oxidized forms of parathyroid hormone with biological activity—Separation and characterization of hormone forms oxidized at methionine 8 and methionine 18. J. Biol. Chem. 1984;259:5507–5513. [PubMed] [Google Scholar]
- 18.Nabuchi Y., Fujiwara E., Ueno K. Oxidation of recombinant human parathyroid hormone: Effect of oxidized position on biological activity. Pharm. Res. 1995;12:2049–2052. doi: 10.1023/a:1016281031373. [DOI] [PubMed] [Google Scholar]
- 19.Reviewer guidance – Validation of chromatographic methods, Center for drug evaluation and research (CDER), November 1994.
- 20.United States Pharmacopeia, 29th ed., United States Pharmacopeial Convention, Inc, Twinbrook Parkway, Rockville, MD, USA, 2006, pp. 1623, 2675–2693, 3050–3053, 3392–3394.
- 21.ICH harmonized tripartite guideline, Validation of Analytical Procedures: Text and Methodology, Q2-R1, 2005.