Abstract
Invasive fungal infections continue to appear in record numbers as the immunocompromised population of the world increases, owing partially to the increased number of individuals who are infected with HIV and partially to the successful treatment of serious underlying diseases. The effectiveness of current antifungal therapies — polyenes, flucytosine, azoles and echinocandins (as monotherapies or in combinations for prophylaxis, or as empiric, pre-emptive or specific therapies) — in the management of these infections has plateaued. Although these drugs are clinically useful, they have several limitations, such as off-target toxicity, and drug-resistant fungi are now emerging. New antifungals are therefore needed. In this Review, I discuss the robust and dynamic antifungal pipeline, including results from preclinical academic efforts through to pharmaceutical industry products, and describe the targets, strategies, compounds and potential outcomes.
As modern medicine advances and allows us to manage diseases that have previously been refractory to medical and surgical intervention, the patient can become vulnerable to invasion by microorganisms. The use of anticancer chemotherapies, new monoclonal anti bodies with immunological properties, immunosuppressive drugs, broad-spectrum antimicrobials that affect our microbiome and numerous medical devices can dysregulate or breach the immune surveillance system. Within this opportunistic window during which individuals are immunocompromised, they are susceptible to invasion by certain fungi.
It has been estimated that there are more than 5 million fungal species worldwide; approximately 300 fungal species have been recorded to cause disease in humans, but only 20–25 of these do so on a relatively frequent basis (TABLE 1). Most of these fungal infections are not transmissible from person to person and do not routinely affect healthy individuals. With the exception of dermatophytic fungal nuisances of the skin and nails, fungal infections are underappreciated by the general public. However, invasive mycoses are a growing problem for clinicians to manage in many medical settings1. In the clinical climate of an ‘at-risk’ population of immunosuppressed hosts that is increasing in size owing to modern medical treatment, particularly of HIV infections, combined with outbreaks of disfiguring mycetomas in some economically disadvantaged societies, these invasive and superficial mycoses have become a major public health problem, with substantial associated morbidities and mortalities. For example, at the peak of the HIV epidemic in the early 2000s, it was estimated that there were more than 1 million cases per year of disseminated cryptococcosis, and an estimated 600,000 of these cases resulted in death2. These alarming figures have decreased with the widespread distribution of effective antiretroviral treatments, which has reduced the spread and disease progression of HIV, but the number of infections still remains very high, particularly in areas of sub-Saharan Africa. On another medical front, aggressive chemotherapy to manage life-threatening cancers is increasingly being used in countries with limited health-care resources, and the resulting increase in life-threatening complications of invasive mycoses will follow, which presents another challenge for these health-care systems. Even highly resourced countries continue to be challenged by the diagnosis and management of invasive mycoses in the at-risk populations. Invasive mycoses can change the landscape of outcomes for certain underlying diseases.
Table 1.
Common invasive fungal diseases
| Disease | Fungal species | Clinical outlook | Treatment options (in order of preference) | Areas for improvement |
|---|---|---|---|---|
| Dimorphic mycoses |
|
|
Azoles > polyenes |
|
| Disseminated cryptococcosis |
|
|
Amphotericin B in combination with 5-flucytosine > monotherapies | Need improved management of IRIS and ICP |
| Invasive aspergillosis |
|
|
Azoles > polyenes > echinocandins |
|
| Invasive candidiasis |
|
|
Echinocandins > azoles > polyenes |
|
| Mucormycosis |
|
|
Polyenes > azoles |
|
ART, antiretroviral therapy; ICP, intracranial pressure; IRIS, immune reconstitution inflammatory syndrome.
It is important to note that our high mammalian body temperature, combined with a powerful immune surveillance system, means that we routinely resist fungal challenges, and primary fungal infections or diseases are an uncommon clinical phenomenon. Furthermore, human mycoses generally result from an accidental encounter with a fungus — such as inhalation of aerosolized spores or direct inoculation with yeasts — and these fungi, which proliferate within immunocompromised human hosts, have access to plenty of carbon and nitrogen sources. Most sites of the human body can potentially provide a safe environment for fungi if immune surveillance is low.
Clinicians have developed several strategies to meet this new invasive fungal infection challenge. First, they can categorize patients into certain at-risk groups to allow more focused strategies for diagnosis and prophylactic, empiric and pre-emptive therapies. Second, the diagnostic toolbox for fungal infections has been substantially improved and includes crucial histopathology of infected tissues when available. Culture techniques are validated and robust, and now with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI–TOF MS), PCR and T2 MRI technology, along with advances in genomic sequencing, the invading fungus can be rapidly and accurately identified. Furthermore, biomarkers, such as the levels of the fungal polysaccharides β-D-glucan, galactomannan and mannan in the blood or other body fluids, have been validated as potential surrogates to help prescribe pre-emptive therapy. These biomarkers enable invasive mycoses to be controlled at the early stages of infection when the tissue burden of fungi is low, an important feature of effective fungal control. Third, there has been a collation of both objective and subjective opinions into a series of antifungal management guidelines3–8, so that health-care systems and clinicians have basic standards to help initiate and compare successful treatments and strategies. Last, the most important factor for the successful management of fungal infections is the continued development and appropriate use of both new and old antifungal drugs.
In the United States, the enthusiasm for antifungal drug development may have been further stimulated by the GAIN Act, the Orphan Drug Act and the Fast Track designation by the US Food and Drug Administration (FDA), all of which potentially apply to antifungal agents and thus provide a favourable economic climate for investment in newly discovered and developed antifungal agents9. However, even with these incentives at both the basic and developmental levels of discovery that are discussed in further detail in this Review, there remains some belief that the development of antifungal agents will be difficult. A key reason for this scepticism is that approximately 80% of antifungal targets in the literature turn out to be false positives with little potential to develop target-based inhibitors with desirable features from them10. Both humans and fungi use similar eukaryotic machinery, and — despite a plethora of potential targets — there needs to be substantial selectivity and few or no complicating interactions with host proteins and the cellular machinery.
What issues and needs are specific to antifungal drug development11? First, mortality that is attributable to fungal infections simply remains too high with current antifungal agents. Second, there needs to be a greater emphasis on more rapid fungicidal activity by new antifungal agents. For improved clinical outcomes, drugs must kill yeasts or moulds rapidly and completely. Currently, the general treatment course for common antifungal agents is too long and thus introduces the potential for poor immediate-term fungicidal activity, reduced patient compliance and/or tolerability, or even the emergence of direct antifungal drug resistance12 (BOX 1). Third, there is a need to widen the spectrum of antifungal activity against some emerging, highly drug-resistant fungi (such as Lomentospora (Scedosporium) prolificans and Candida auris) that are increasingly observed in patients who are being immunosuppressed for other severe underlying diseases and are thus admitted to hospitals. There are simply no antifungal agents that can adequately treat infections caused by certain fungal strains. Fourth, it is important to have an optimized combination of therapeutic agents or classes to increase potency and reduce the emergence of drug resistance. Fifth, resistant strains are increasing in number for some antifungal agent classes, particularly for the azoles and the echinocandins13. Sixth, safety remains a very important issue for antifungal drug use. Invasive mycoses occur in very fragile patients who cannot tolerate much additional organ toxicity from other treatments. Furthermore, these patients are frequently taking multiple other therapeutic agents, so drug–drug interactions need to be carefully considered. Last, there are multiple issues around the clinical development of new antifungal drugs. Despite the importance of and need for new antifungal agents, successful clinical drug development has a series of roadblocks that need to be overcome (BOX 2). All of these specific issues or difficulties can be managed, but it takes an experienced team with insightful protocols and careful management of the trial to carry out and complete these licensing studies.
Box 1. Resistance to current antifungal agents.
The mechanisms that have been acquired by fungi to become resistant to current antifungal agents are fairly well understood. It is important to note that, unlike bacteria, there are no known plasmid- or transposon-mediated mechanisms for drug resistance. For instance, polyene resistance is rarely acquired; most resistance to polyenes is primary and thus observed in fungal species that have ergosterol membranes that are not susceptible to or only mildly affected by polyenes. However, polyenes can lose their fungicidal activity during prolonged exposure of the fungus to the drug, which leads to reduced efficacy or clinical resistance144.
Azoles, by contrast, have multiple mechanisms for the development of both primary and acquired resistance, including mutations in the gene encoding lanosterol 14α-demethylase (ERG11), so that azoles can no longer block its catalytic activity, and amplification of ERG11, so that Erg11 molecules overwhelm the inhibitory capacity of the azole145. Also, some fungal species have amplified or induced efflux pumps to remove azoles from the fungal cell and therefore from the target. This rise in azole resistance has been observed consistently in hospitals for years and has even been linked, in certain cases, to the environmental use of fungicides in agriculture146.
Over the past 5–6 years and with the widespread use of echinocandins in hospitals, there has clearly been an increase in the identification of yeasts that have become resistant to echinocandins, through the development of mutations in FKS1, which encodes the 1,3-β-D-glucan enzyme that helps in the formation of the fungal cell wall. This echinocandin resistance mechanism has been most prominently observed in the haploid yeast, Candida glabrata147.
Recently, mutations in MSH1, a mismatch repair gene, have also been observed to cause multiple drug-resistant phenotypes (to azoles and echinocandins) in yeast strains148. Finally, drug-resistant mutations in the pyrimidine pathway occur at the rate of 1 in every 106–107 yeast cells, so 5-flucytosine is very vulnerable to the development of drug resistance if used as a monotherapy in high fungal burden infections149.
Box 2. Antifungal drug development considerations.
A number of hurdles to antifungal drug development must be overcome and will determine the clinical utility of these compounds and the development path:
The value of limited- versus broad-spectrum antifungal activity must be balanced. For instance, a drug with antifungal activity only against particular yeasts or moulds will rely heavily on diagnostic acumen
For eukaryotic targets, the toxicity data will always be crucial
Biomarkers should be accepted as validated primary end points instead of relying exclusively on death: these patents have too many other comorbidities, so efficacy signals will require large number of patients
Although there are substantial numbers of invasive fungal infections, many patients will often need to be screened to get a single patient enrolled. This is particularly true for candidaemia studies
The use of compounds for prophylaxis or for treatment should be considered as an initial focus for the development of safe and broad-spectrum compounds
Although more rapid fungicidal activity and shorter treatment courses are needed, initial studies will probably require prolonged treatment duration
Invasive fungal infections classically occur in patients with the most severe illnesses, so both underlying diseases and tolerability can frequently affect the outcome assessment of the antifungal drug
Many of the scoring systems use radiographic results, and this is simply too imprecise
Success should include disease control, rather than disease eradication, in many circumstances
The clinical studies are particularly expensive. These patients are fragile and require close monitoring as well as detailed records. Furthermore, there may be interruptions of assessments due to decisions by the clinical care team
It is truly an appropriate time to review antifungal drug development, and to discuss its current leads and directions, so that this important antifungal pipeline can be re-evaluated14–16. In this Review, I examine the currently available antifungal agents and provide an overview of the ongoing efforts to develop completely new classes of drugs, which includes strategies to repurpose existing drugs, develop new drugs from current antifungal classes and create new biological antifungal agents.
Currently available antifungal drugs
For many of the superficial mycoses, there have been a series of topical drugs used over the past century that have had moderate success in controlling common and irritating infections. By contrast, for the invasive mycoses, which require systemic antifungal therapy, there have been only four major classes of antifungal agents (polyenes, flucytosine, azoles and echinocandins) (FIG. 1).
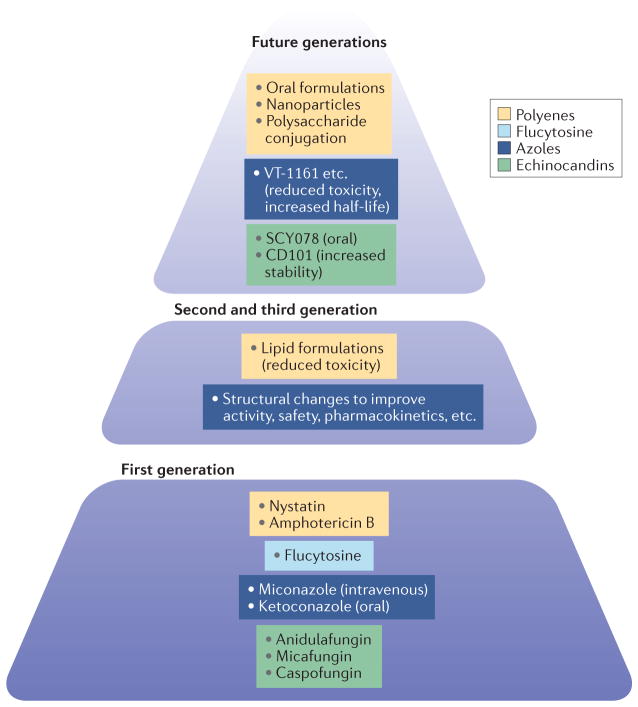
Figure 1. Currently available antifungal compounds and future derivatives thereof.
There are currently four classes of antifungals available: polyenes, flucytosine, azoles and echinocandins. Improvements have been made to three of these classes to increase efficacy or reduce toxicity. Future generations of these classes of compounds are in development.
A central feature of antifungal agents is the requirement for the eukaryotic machinery to be differentially blocked or destroyed in the fungus but do limited or no damage to host cellular functions17. The lineage of successful systemic antifungal drugs started in the 1950s with the approval of the polyene amphotericin B deoxycholate. Despite its substantial toxicities, amphotericin B, in formulation with deoxycholate or other solubilizing agents, remains the most potent fungicide and has the broadest antifungal spectrum of the systemic antifungal agents in clinical use today. The polyenes interact with ergosterol-containing fungal membranes and disrupt them by puncturing these membranes (FIG. 2), but they can also interact with cholesterol-containing membranes and thus injure host cells, so host toxicity has always been an issue. In the mid-1990s, the formulation of amphotericin B was vastly improved with the creation of lipid formulations of amphotericin B, such as AmBisome and amphotericin B lipid complex, that reduced host toxicity, especially the occurrence of renal dysfunction.
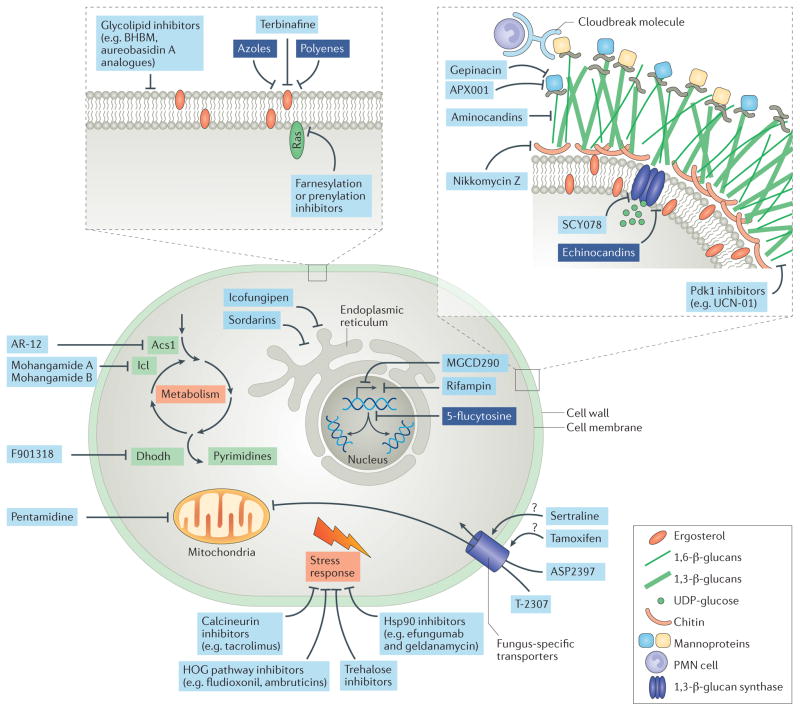
Figure 2. Antifungal targets.
Numerous molecules can be attacked by antifungals, including fungus-specific components of the cell wall or cell membrane, or processes such as metabolism, DNA synthesis, mitochondrial function or the stress response. Investigational antifungal agents targeting these components are indicated in light blue boxes, and approved antifungal classes are indicated in dark blue boxes. Some antifungals exert their specificity by being taken up by fungus-specific transporters. Acs1, acetyl-CoA synthetase 1; BHBM, N′-(3-bromo-4-hydroxybenzylidene)-2-methylbenzohydrazide; Dhodh, dihydroorotate dehydrogenase; HOG, high-osmolarity glycerol; Hsp90, heat shock protein 90; Icl, isocitrate lyase; Pdk1, 3-phosphoinositide-dependent protein kinase 1; PMN, polymorphonuclear; UDP, uridine diphosphate.
Flucytosine is a pyrimidine analogue that was approved in the 1960s and is generally used in limited circumstances, such as in combination with a polyene for cryptococcal meningitis. Flucytosine is rarely used alone because of the rapid development of drug resistance that occurs during monotherapy. Flucytosine is converted by a cytosine deaminase that is not present in humans to the toxic compound 5-fluorouracil, which interferes with RNA and DNA metabolism.
In the late 1970s, the systemic azoles started their ascension to become a first-line choice for the treatment of invasive fungal infections. The azoles primarily block ergosterol synthesis by inhibiting lanosterol 14α-demethylase (Erg11), a primary target in the fungal membrane that is not present on the host cell membrane. The first azole-based therapies were an intravenous miconazole preparation and an oral ketoconazole tablet. The second generation, the extended-spectrum azoles, improved on the spectrum of antifungal activity, safety and pharmaco kinetics, and were available in new formulations. For instance, fluconazole and itraconazole have both intravenous and oral formulations. Following further discovery and development, there are now third-generation extended-spectrum azoles: voriconazole, posaconazole and isavuconazole. The merits of these newer azoles are substantial as they have improved antifungal activity, safety, pharmaco kinetics and formulations. They are a key component of the current clinical management of invasive mycoses as wide-spectrum antifungal agents for use in prophylaxis (to prevent infection), and pre-emptive (to prevent disease manifestations), empiric and therapeutic strategies. The azoles have been shown to reduce mortality and allow clinicians to practise aggressive medical and surgical management of many serious underlying diseases18.
The development and marketing of the fourth class of antifungal agents, the echinocandins, began in the early 2000s. The echinocandins block 1,3-β-glucan synthase, which helps to form the cell wall (a unique structure of the fungus that is not present in mammals). Indeed, the echinocandins have very little host toxicity, and this is one factor that contributes to their frequent use in the hospital setting. Three echinocandins (caspofungin, micafungin and anidulafungin) have had much success over the past decade in the clinic owing to their broad-spectrum fungicidal activity against yeasts, their reduced drug–drug interactions and their improved safety. This antifungal class has become the first line of treatment for the deadly and relatively common nosocomial candidaemia and invasive candidiasis4.
Finally, the squalene epoxidase (also known as squalene monooxygenase) inhibitor terbinafine is used, albeit uncommonly, as a combination agent in invasive fungal infections, and aerosolized pentamidine is infrequently used in prophylaxis against pneumocystis infections.
There have been several excellent detailed discussions of the mechanisms of the antifungal agents in use at present18–22. Each currently used class has both strengths and weaknesses in terms of efficacy and safety. These weaknesses are clearly emphasized by the mortality rates attributable to invasive mycoses, which remain substantial. For instance, in invasive candidiasis, the mortality rate has been estimated to be around 40%23; the mortality rate among individuals with disseminated cryptococcosis is 20–30% in well-resourced health-care systems24, and this rate is substantially higher in resource-limited areas (50% or greater)25. By contrast, the mortality rate among individuals with invasive aspergillosis has been reduced over the past decade but has now plateaued at approximately 20%26. In many other invasive mould infections, the reported mortality rates are much higher (≥50%). Although a new azole, isavuconazole, was approved for treatment of aspergillosis and mucormycosis in 2015, it has been more than a decade since a new class of antifungal agents has been introduced into the clinic.
Novel pathways and targets
Over the past two decades — during the fungal genomic era — there has been substantial progress in antifungal drug development for a multitude of potential drug targets27 and inhibitors28,29. Notably, antifungal molecules are frequently discovered in phenotypic screens for antifungal inhibitors, and these strategies require live fungus readouts, natural products as sources of molecules and pharmaceutical insights to move identified molecules forward30–34. This Review focuses on several examples of promising pathways and specific targets (TABLE 2, FIG. 1) but is not comprehensive. These specific pathways were chosen as prime examples of the mechanistic approach to block important fungal molecules. The following specific target studies illustrate the depth and the potential for the detailed, mechanistic approaches to antifungal target development at an early exploratory stage as a foundation for the future anti fungal drug pipeline.
Table 2.
Antifungal compounds with novel targets in development
| Compound | Molecular target | Target species or relevant disease | Development stage | ClinicalTrials.gov identifier or ref. |
|---|---|---|---|---|
| Unapproved compounds with novel structures | ||||
| APX001 | Glycosyl phosphatidylin-ositol synthesis (prevents attachment of mannose proteins to the outer cell wall) |
|
Phase I; phase II planned | NCT02957929 and NCT02956499 |
| AR-12 |
|
|
Completed phase I for oncology indications | NCT00978523 |
| ASP2397 | Unknown, but taken up by Sit1 |
|
Preclinical but potentially approaching clinical studies | – |
| Aureobasidin A | Inositol phosphorylceramide synthase | Broad spectrum | Preclinical | – |
| Cloudbreak molecules (bispecific antibodies) | Binds to granulocytes and thereby concentrates the antifungal at the infection | Could have focused or broad-spectrum antifungal activity | Preclinical | – |
| Efungumab (also known as Mycograb) | Hsp90 | Candida spp. | Phase II | NCT00324025 and NCT00847678 |
| F901318 | Dihydroorotate dehydrogenase |
|
Phase II | NCT02856178 |
| Geldanamycin-like agents | Hsp90 | Broad spectrum | Completed phase I for oncology indications | 148 |
| MGCD290 | Hos2 |
|
Phase II | NCT01497223 |
| Nikkomycin Z | Chitin synthase |
|
Phase I | NCT00834184 |
| T-2307 | Mitochondrial membrane potential |
|
Phase I (see Further information for details) | – |
| Compounds that could be repurposed | ||||
| Cyclosporine, tacrolimus or rapamycin | mTOR or calcineurin |
|
Approved for post-transplant immunosuppression | – |
| Rifampin | RNA polymerase |
|
Approved for bacterial infections | – |
| Sertraline | Serotonin reuptake |
|
Approved for depression | – |
| Tamoxifen | Oestrogen receptor |
|
Approved for oestrogen receptor-positive breast cancer | – |
| Verapamil | Calcium channel |
|
Approved for cardiac conditions (including hypertension and arrhythmias) | – |
Hos2, histone deacetylase 2; Hsp90, heat shock protein 90; mTOR, mechanistic target of rapamycin; Sit1, siderophore iron transporter 1.
Calcineurin
The calcineurin pathway is important in eukaryotes and is potentially a target of selective inhibitors that could become antifungal drugs35,36. The serine/threonine phosphatase calcineurin (also known as protein phosphatase 3; a hetero dimer that is composed of the subunits calcineurin A and calcineurin B) is the target for the most commonly used transplant anti-rejection drugs: tacrolimus and cyclosporine. Cyclosporine was initially discovered in a screen as an anti-Candida molecule, and calcineurin is central to the stress responses of many pathogenic fungi. For instance, calcineurin has been shown to be essential for the virulence composite of the major fungal pathogens (Aspergillus fumigatus, Candida albicans and Cryptococcus neoformans) and thus for fungal survival and fitness in the host37. Furthermore, calcineurin can be linked to other well-studied stress signalling pathways, including the heat shock protein 90 (Hsp90)38 pathway. Hsp90 has also become an excellent target for antifungal inhibitors, and an antibody against Hsp90 (efungumab; also known as Mycograb; NeuTec Pharma/Novartis) in combination with amphotericin B made it into a substantial, comparative clinical trial and demonstrated therapeutic efficacy in invasive candidiasis39. The potential to make calcineurin a viable target for antifungal drug discovery has been contingent on finding specific inhibitors that have the differential ability to block fungal over mammalian calcineurin. In this respect, the inhibitors must be fungicidal with minimal or no immunosuppressive activity. It has been helpful that the fungal and human homologues of calcineurin are well understood from a structural perspective36. It is with this knowledge and a concerted chemistry effort that progress is being made in discovering potent antifungal, anti-calcineurin molecules with reduced immuno suppressive activity; these are currently being tested in animal models40.
Sphingolipid synthesis and RAS
Two pathways (RAS41 and sphingolipid synthesis42) have been active areas in the search for new anticancer agents. However, genetic and structural studies have also shown that both of these signalling pathways are important for fungi to efficiently produce disease. Fungal sphingolipids have emerged as a potential target for new antifungal agents because the biosynthesis of these molecules in fungi is structurally different from that in mammals. Sphingolipids are important in cellular metabolism but can also be important in the regulation of the fungal virulence composite. There are now diverse strategies that could be used to block the synthesis and/or function of sphingolipids43. Several new compounds (N′-(3-bromo-4-hydroxybenzylidene)-2-methyl benzohydrazide and its derivative, 3-bromo-N′-(3-bromo-4-hydroxybenzylidene) benzohydrazide) have been found to selectively decrease levels of fungal glycosphingolipids relative to the mammalian ones. These compounds are anticipated to go into clinical trials (see the American Society for Microbiology press release). In the RAS pathway, there are several antifungal targets within the protein farnesylation and prenylation processes that could be of interest, as the structures differ between the fungal and mammalian proteins41. With these observations and the ability to design and/or discover molecules that would inhibit the actively growing fungi but have no or little effect on human cells, a potent antifungal agent could emerge. Similarly to the calcineurin inhibitors, these targets have been validated with inhibitors for the human enzymes during the development of potential anticancer agents. Therefore, the focus will be on finding inhibitors with selectivity for the fungal over the human homologues and, importantly, on getting the molecules to the site of action within the intact fungal cells.
Trehalose
The trehalose pathway comprises several enzymes that are connected to glycolysis and creates the regulatory molecule trehalose-6-phosphate and the disaccharide sugar trehalose44. Relative to compounds targeting the RAS and calcineurin pathways, molecules targeting this pathway are at a much earlier developmental stage, and few inhibitors have been discovered thus far. Despite this lack of development, many targets within the trehalose pathway possess attractive features of antifungal agents. First, unlike many of the fungal stress pathways that are currently being studied, this pathway is present in fungi, bacteria, plants and invertebrates, but is not found in the mammalian biochemical machinery. Therefore, the potential for inhibitor toxicity should be minimal, unless there are off-target issues. Second, this pathway, which contains two primary synthesizing enzymes (trehalose-6-phosphate synthase (Tps1) and trehalose-6-phosphate phosphatase (Tps2)), has been validated as essential to tolerating stress in all of the major fungal pathogens44. Third, genetically blocking this pathway kills pathogenic fungi, which clearly demonstrates that this pathway is a fungicidal target45,46. Recently, the crystal structures of the C. albicans proteins Tps1 and Tps2 have been solved47, so molecules that specifically bind to active catalytic components to inhibit the enzymes could be found computationally or in compound libraries47.
Other targets
Several other pathways have been identified as potential antifungal targets. For instance, the metabolic glyoxylate cycle, specifically the enzyme isocitrate lyase, is important for C. albicans and A. fumigatus invasion but not C. neoformans invasion, and has been a target of some potent inhibitors, such as mohangamide A and mohangamide B, which inhibit the enzyme in C. albicans48. The mitogen-activated protein (MAP) kinase and the high-osmolarity glycerol (HOG) pathways, which are required for adaptation to environmental signals, have also been attractive targets. The HOG pathway was identified as the target for the antifungal action of fludioxonil49–51. New targets and inhibitors also include the cell wall target 3-phosphoinositide-dependent protein kinase 1 (Pdk1) and an inhibitor, VCN-01 (REF. 29), of calcium signalling. The sphingosine-1-phosphate receptor modulator, fingolimod hydrochloride (also known as FTY720), also has broad-spectrum antifungal activity52. Finally, even altering gene expression through the manipulation of transcription factors has emerged as an attractive antifungal approach: a prime example of this strategy has been the successful identification of compounds that block the transcription factor sterol uptake control protein 2 (Upc2)53.
The above pathways are examples of potential antifungal targets but do not constitute an exhaustive list. However, they are clear examples of how academic research can provide the framework to discover and validate antifungal targets and thus provide a platform to identify and develop antifungal molecules. From these robust scientific platforms for targets and inhibitors, the pharmaceutical industry can then use their chemistry infrastructure, formulation expertise and molecule libraries to identify and/or further develop antifungal drugs. To target these ubiquitous and highly linked pathways, the ideal compounds should have a broad antifungal spectrum, not overlap with existing drug-resistant mechanisms and potentially synergize with either new or old antifungal drugs. The main focus of these studies must remain on the identification of powerful fungicidal targets that will work within the host, as the final goal is to identify and develop new drugs that are clinically useful.
There also remains a robust platform for the discovery of natural products with antifungal activity, and many natural antifungal compounds have been reported. For instance, psoriasin (also known as S100A7), a mammalian protein that is commonly found in psoriatic lesions, has antifungal activity, particularly against Trichophyton rubrum54. Other products such as humidimycin (also known as MDN-0010), a bacterial natural product, potentiate known antifungal agents by targeting an echinocandin salvage pathway55. Although nature can help to select antifungal compounds, we must then make them into drugs. From impressive antifungal scaffolds56 and screening of natural products57 to finding natural products such as carvacrol58 and the antibacterial quinolone analogues that are active leads against members of the fungal kingdom59, the strategies for antifungal compound discovery from natural products are diverse and robust.
New agents in development
Multiple types of antifungal molecules are in clinical development and currently have substantial support from pharmaceutical companies. For a variety of reasons, three groups of antifungal compounds — the aminocandins60,61, sordarins (which inhibit protein synthesis by stabilizing the ribosome–elongation factor 2 (Ef2) complex)62 and icofungipen (which is an isoleucyl-tRNA synthesis inhibitor) 63 — have received little recent developmental attention, and a broad-spectrum triazole, albaconazole64, is now only studied for its use in treating superficial fungal infections, whereas before it was considered a potential compound for the treatment of systemic fungal diseases65. Therefore, these compounds are not emphasized in this Review. The focus of this section is on the targets and mechanisms of action of the compounds, the general antifungal susceptibility pattern of the compound and its preliminary in vivo activities (TABLE 2).
ASP2397
A new antifungal compound, ASP2397 (Vical), produces its antifungal effects by disrupting the intracellular fungal biochemical machinery. Its precise target inside the fungal cell remains uncertain, but ASP2397 localizes within fungi such as A. fumigatus, as it is taken up by the specific siderophore iron transporter 1 (Sit1)66. As mammalian cells do not have this transporter, it is hypothesized that the compound will have excellent selective fungal toxicity. In both in vitro and in vivo studies, ASP2397 has very potent fungicidal activity against A. fumigatus67. Along with potent activity against both azole-susceptible and azole-resistant strains of Aspergillus spp., it also has in vitro antifungal activity against a few Candida spp. and some rare moulds and yeasts, such as the Fusarium spp. and Trichosporon spp.66.
T-2307
The allylamine T-2307 (Tokuyama Corporation) has been in development for several years. It has interesting and unusual mechanisms for selectivity and activity. First, the compound is selectively transported and accumulates in fungal cells through a specific polyamine transporter68. Once inside the yeast cell, it specifically inhibits the mitochondrial membrane potential, and this has profound fungicidal activity, as the fungal strains that cause human disease are primarily respiratory fungi69. This compound has broad-spectrum and potent antifungal activity against Candida spp., Cryptococcus spp. and Aspergillus spp., with extremely low minimum inhibitory concentrations for yeasts and moulds70. Furthermore, the in vitro antifungal activity is corroborated by impressive antifungal activity in the treatment of animal models of mycoses. In some models, T-2307 is more potent than standard azoles and polyenes for the treatment of mycoses71. However, for unclear reasons, it has yet to be advanced into clinical trials.
AR-12
The celecoxib derivative AR-12 (Arno Therapeutics) is a repurposed compound in some respects. It was first used in phase I oncology trials, so safety has been determined in humans (ClinicalTrials.gov identifier: NCT00978523). However, it was shown to have consistent antifungal activity against yeasts, such as C. neoformans and C. albicans, and moulds, including those from the Mucorales order and the hyalohyphomycosis group, such as Fusarium spp. and Scedosporium spp.72,73. In animal models, AR-12 potentiates the activity of fluconazole against C. neoformans infection72. The mechanism of action of this compound is not precisely known. AR-12 seems to act via two mechanisms: by blocking acetyl-CoA synthetase 1 in fungal metabolism and by downregulating host chaperone proteins, such as 78 kDa glucose-regulated protein (GRP78), HSP90 and HSP27 (REF. 73), which reduces the host immune response. Targeting the host immune response may provide a high genetic barrier to the development of resistance compared with the direct antifungal activity of most agents used for the treatment of invasive fungal infections.
F901318
F901318 (F2G) is a member of the new orotomide class of antifungal agents. It is currently in phase II development as an intravenous and oral agent for use in systemic mould infections, with a particular emphasis on aspergillosis and scedosporiosis (NCT02856178)8. The mechanism of action is very well described. It is a potent inhibitor of Aspergillus spp. dihydroorotate dehydrogenase, which is crucially involved in fungal pyrimidine biosynthesis8. Humans also have this enzyme, but F901318 is a 2,000-fold more potent inhibitor of the fungal enzyme than the mammalian enzyme homologue and, with this differential inhibitory activity, its toxicity towards mammalian cells is anticipated to be very low8. A similar phenomenon had previously been observed with an antibacterial compound, trimethoprim, which inhibits dihydrofolate reductase in both bacteria and humans with substantially different kinetics. F901318 has potent anti-Aspergillus activity in treatment of animal models8. Although it has poor activity against yeasts in vitro, it does possess fungicidal activity against a series of moulds and most endemic (dimorphic) mycoses8.
APX001
The first-in-class compound APX001 (also known as E1211; Eisai) is an antifungal compound that has been transferred to Amplyx Pharmaceuticals for further development. APX001 is a prodrug that is converted to the active moiety E1210 in the presence of alkaline phosphatase in vivo. This compound is an inhibitor of glycosyl phosphatidylinositol (GPI) synthesis and thus hinders the attachment of essential adhesion proteins (such as mannoproteins) to the outer fungal cell wall, a process that is mediated by GPI-anchored wall transfer protein 1 (Gwt1)74,75. Another GPI inhibitor (gepinacin) that also blocks Gwt1 has recently been discovered76. These GPI-anchored proteins provide cell wall integrity and are involved in membrane homeostasis, and fungal mannoproteins promote adhesion, pathogenicity and immune evasion. APX001 specifically inhibits the conversion of glucosaminylphosphatidyl inositol to its acrylate form, glucosaminyl(acyl)phosphatidyl inositol, an essential step in GPI synthesis75. Furthermore, APX001 has excellent, broad-spectrum potency with antifungal activity in vitro against Candida spp. and Aspergillus spp., and can provide antifungal activity against some drug-resistant yeasts and moulds77,78. It even has potent antifungal activity in vitro against moulds that are difficult to treat, such as Fusarium spp. and Scedosporium spp.79. Finally, APX001 has some direct antifungal activity against the Mucorales in vitro79. In animal models, APX001 has potent and consistent fungicidal activity, and can be additive or synergistic with other antifungal agents, such as the azoles or echinocandins77. At present, APX001 is in phase I (NCT02956499 and NCT02957929), and phase II clinical trials are planned.
Nikkomycin Z
Nikkomycin Z has a protracted history, and its development might have been delayed by its poor antifungal activity as a stand-alone agent against yeasts such as Candida spp.. It was first discovered in the 1970s by Bayer. Shaman Pharmaceuticals held the rights to its development during the 1990s, but the University of Arizona, which still has this compound in development, obtained those rights in 2005 (REF. 80). Nikkomycin Z resembles uridine diphosphate (UDP)-N-acetyl glucosamine, which is a precursor of a major component of the fungal cell wall, chitin. Nikkomycin Z is a competitive inhibitor of chitin synthase. It has been a very potent fungicidal agent for the treatment of murine coccidioidomycosis, histoplasmosis and blastomycosis81, and as its target is a component of the cell wall, it has additive or synergistic in vitro and in vivo activity with the 1,3-β-glucan synthase inhibitors (echinocandins)82. As nikkomycin Z targets the fungal cell wall, it is likely to be safe for clinical use. Its ability to potentiate the antifungal activity of echinocandins makes nikkomycin Z plus an echinochandin an ideal broad-spectrum drug combination to attack the fungal cell wall in both yeasts and moulds. Furthermore, new chemistry studies of the peptidyl nucleoside antibiotics, which includes nikkomycins and polyoxins, may enable further development of this class of compounds83.
MGCD290
The histone deacetylase 2 (Hos2) inhibitor MGCD290 (Mirati Therapeutics) is effective in combination with both azoles and echinocandins in vitro and in animal models84,85. Histone deacetylases remove acetyl groups from lysines on core histones, HSP90 and other cellular proteins, and thus have important roles in the regulation of gene transcription and control other cellular functions, such as cell proliferation and death. Hsp90 is a molecular chaperone that regulates crucial cell responses to both cell membrane and cell wall stresses86, so inhibiting Hsp90 could help to block the cellular stress responses and thus potentiate standard cell wall or membrane inhibitors. The stress response in the fungal cell is mediated by both Hsp90 and its client protein calcineurin. Therefore, blockade of either of these targets could be synergistic with other inhibitors, such as azoles or echinocandins. Much work has been carried out to investigate HSP90 inhibitors, such as the geldanamycin-like agents38,87 and the monoclonal antibody efungumab39,88,89. Notably, the capacity of MGCD290 to synergize with known antifungals is retained even when there is some apparent resistance to the primary, established drug84. This compound has the potential to be a potent enabler to increase fungicidal activity, overcome resistance and broaden the activity of other antifungal drugs. However, in the first human trial in severe vulvovaginal candidiasis, combining MGCD290 with fluconazole did not give better results than fluconazole treatment alone (see MethylGene press release). Recapitulating the positive results observed during testing in vitro and in animal models can be challenging in the human host for a variety of reasons, including differing pharmacokinetics, burden of fungus at the site of infection and host immune responses.
Aureobasidin A
Aureobasidin A is a very potent natural product inhibitor of an essential and unique enzyme, inositol phosphorylceramide synthase, which catalyses a pivotal step in fungal sphingolipid biosynthesis90. This enzyme has been a broad-spectrum antifungal target, and potent antifungal aureobasidin A analogues have been generated through medicinal chemistry90,91. AureoGen Biosciences has recently signed a licensing agreement with Merck & Co. for novel derivatives of aureobasidin A that are made with AureoGen’s chemistry platform, and these have improved antifungal activity against yeasts and moulds. Inositol phosphorylceramide synthase and its natural product inhibitors have attracted renewed therapeutic interest.
Targeted delivery approaches
A very new and evolving concept in cancer therapies has been adopted by the antifungal discovery field: targeting a drug to the relevant site. Small bispecific molecules have been made using Cloudbreak technology (Cidara Therapeutics). These compounds comprise an effector moiety that attaches to host cells (such as granulocytes), which enables the compound to accumulate at the site of infection, and an echinocandin moiety that attaches to fungal cell walls and exerts antifungal activity. Therefore, this bispecific molecule attaches to granulocytes and is taken to the site of infection, where it accumulates in high concentrations near the fungus and exerts direct antifungal effects. In a related approach, T cells have been engineered to express chimeric T cell receptors that contain extra cellular dectin 1 (also known as CLEC7A) domains and thus become activated upon interaction with the fungal cell wall, thereby localizing cytotoxic immune activity to fungus- infected sites. Infusion of these dectin 1–chimeric antigen receptor (D–CAR) T cells reduces fungal burden and mortality in mouse models of aspergillosis92. Directing or concentrating antifungals or host immune cells to infection sites, either with bispecific molecules or targeting specific effector host cells, is an area that could identify a potent and creative future fungicidal solution.
Improving existing antifungals
There are four promising initiatives to improve on existing antifungal compounds (FIG. 1). First, for the class of polyenes (for example, amphotericin B and nystatin)93, safer and more effective broad-spectrum drugs are under development. These include changing the structure of amphotericin B from ‘molecular umbrella conjugates’ (REF. 94) to alternative structures, such as nanoparticles95 and conjugated polysaccharides96,97, that may be able to penetrate certain body compartments and reduce membrane toxicity97,98. One proposed mechanism for this reduced toxicity is that the aggregated forms of amphotericin B have very different activities (specifically toxicities) compared with monomers: aggregates target host cells and thereby increase toxicity, whereas the monomers should target fungi preferentially over the host cells99. Another considerable advance in this area has been to produce an oral formulation for polyene treatment. An oral drug delivery formulation consisting of amphotericin B cochleate lipid–crystal nanoparticles (Matinas BioPharma) has shown in vivo activity in animals and is now in clinical trials (NCT02971007 and NCT02629419)100.
In regard to 1,3-β-glucan synthase inhibitors, there are two areas of investigation. First, the echinocandin CD101 (Cidara Therapeutics) has a chemical modification on the echinocandin backbone that makes the compound more stable, and phase II trials (NCT02733432 and NCT02734862) have been initiated for this long-acting echinocandin101,102. The improved stability of the molecule has created two new features for the echinocandins: this drug can now be used as a topical agent for skin and vaginal infections, and the substantially longer half-life in the blood may allow for treatment with weekly dosing. The second area of investigation involves another 1,3-β-glucan synthase inhibitor, SCY078 (also known as enfumafungin; Scynexis), which is in phase II trials for treatment of yeast infections (NCT02679456). This is a triterpene 1,3-β-glucan synthase inhibitor, and its primary advantage is its oral bioavailability. SCY078 has similar activity against yeasts to that of the echinocandins103 but has some in vitro antifungal activity against echinocandin-resistant yeasts. It should be a safe compound, as it targets the fungal cell wall, a feature that is not present in mammalian cells, and SCY078 also has some antifungal activity against certain moulds87.
In addition, even the widely used and developed azole class of antifungal agents has been further modified with a new chemistry approach, which might have exciting implications for the clinic. The ‘Achilles heel’ of currently available triazoles is that polypharmacy is common in patients with serious illnesses, so many individuals experience negative side effects through drug–drug interactions. The Viamet Pharmaceutical platform uses the metal-binding group of the standard azole compounds. Following proprietary manipulation of this part of the molecule, compounds have now been developed that have substantially reduced interactions with cytochrome P450 and thus fewer potential drug– drug interactions. This platform has yielded several new compounds: VT-1161 (REFS 104,105), which is in phase II clinical trials for onychomycosis106 and vaginal candidiasis (NCT02267356 and NCT02267382), and a very potent anti-cryptococcal compound, VT-1129, which has outstanding efficacy in vitro and in animal models107. Finally, in their portfolio is VT-1598, which is a potent azole, with activity against endemic mycosis (see the Business Wire press release) and cryptococcosis. Viamet’s platform has also generally increased the half-lives of the newly created azoles by chemical optimization of the active antifungal backbone of the triazole, thereby generating potent and broad-spectrum compounds. Through this creative chemical modification of an old class of molecules, a fourth generation of effective antifungal azoles could emerge.
Repurposing old drugs
The discovery of new therapies for fungal infections can be enriched by the ability to identify antifungal activity in approved drugs and to direct them to the antifungal pipeline108. There is an ample history of using established drugs, including antibacterial agents, for fungal infections. For example, although not used in standard clinical practice, the addition of a known antibacterial agent, such as the RNA polymerase inhibitor rifampin, has been examined and shown to enhance the antifungal activity of established antifungal agents in vitro109. Calcium channel blockers, such as verapamil110, also amplify antifungal drug potency. Possibly owing to drug–drug interactions, increased toxicity or a lack of funding for clinical trials, most of these repurposing agents have not had much clinical success, so most remain intriguing hypothetical tools for further antifungal drug development and discovery.
Since the introduction of the fungistatic azole fluconazole more than 25 years ago, the treatment of cryptococcosis has not advanced substantially111. There have been at least three attempts to repurpose old drugs for cryptococcosis infections. First, as previously noted, calcineurin inhibitors have anti-cryptococcal activity both individually and in combination with other compounds112. They are, however, immunosuppressive agents, and cryptococcosis can occur while patients are receiving them. Nonetheless, upon closer inspection of clinical outcomes, patients receiving the calcineurin inhibitor tacrolimus had more skin infections than internal infections with cryptococcosis113, which is consistent with reduced survival of cryptococci at high body temperatures upon blockade of calcineurin function. Clearly, these immunosuppressive compounds (cyclosporine, tacrolimus and rapamycin) will need to be manipulated to reduce immunosuppression, but if this group of drugs could be modified to be more fungicidal and less immunosuppressive, an effective antifungal drug could be generated.
Second, studies carried out more than 50 years ago showed that an oestrogen, diethylstilbestrol, had in vitro anti-cryptococcal activity. Attempts have been made to show in vivo activity, but it was not clear whether this hormonal approach would work114,115. However, recent studies have demonstrated that a compound used to treat breast cancer, the oestrogen receptor-targeting drug, tamoxifen, has anti-cryptococcal activity and could be combined with fluconazole as an all-oral treatment option to enhance anti-cryptococcal activity116,117.
Third, the most advanced repurposing attempt is the adjunctive use of sertraline in cryptococcal meningitis. Sertraline is a selective serotonin reuptake inhibitor that is primarily used to manage depression. However, sertraline has been shown to potentiate the anti-cryptococcal activity of azoles118. After a successful exploratory phase II study with the use of sertraline as adjunctive therapy for cryptococcal meningitis119, the investigators are approximately half-way through a phase III study to determine the value of adding this compound to a standard induction therapy for cryptococcal meningitis (NCT01802385). This randomized, comparative study is a structural paradigm for how to get these established drugs repurposed.
For many decades, antineoplastic agents have been used to treat eukaryotic hyperproliferation in the form of cancer, although they might not be direct antifungals. Many of these anticancer agents have antifungal activity, and their clinical history can be used by investigators to reduce the toxicity of these drugs while preserving their anticellular or antifungal activity120,121. In a serendipitous way, anticancer compounds, which often cause immunosuppression and thus increase the incidence of fungal infections, could be chemically manipulated to produce congeners that effectively treat these opportunistic fungal infections. For example, miltefosine is an alkyl phosphocholine compound that was initially developed as an anticancer agent but now has indications for treatment of protozoan infections, such as Leishmania infections, in some countries. Miltefosine had been shown to have antifungal activities in vitro and in animal models122. However, studies have also demonstrated little in vivo activity against cryptococcosis123, which makes its use as an antifungal less certain and demonstrates how robust the preliminary studies must be to advance repurposed drugs. On a similar note, a new oral preparation of itraconazole (also known as ‘super bioavailability’ (SUBA)-itraconazole; Mayne Pharma) is under investigation for the treatment of patients with basal cell carcinoma nevus syndrome (NCT02354261). The anticancer properties of itraconazole come from its inhibition of both angiogenesis and Hedgehog signalling124. However, the new nanosuspension formulation has improved bioavailability125, so it might be co-opted again by clinicians for its antifungal activity, and Mayne Pharma could direct efforts towards its development in the antifungal arena. Finally, antifungal agents and basic antiseptic chemicals have been altered to make formulations that can be aerosolized for delivery into the lungs126, including new powder formulations127, or incorporated into formulations that can attack yeast biofilms on foreign bodies and could therefore be used as antifungal solutions or coatings to preserve catheters128.
Host immune cell-targeted approaches
The increased focus on immune diseases and immunooncology has also been helpful for developing strategies for the antifungal pipeline. For example, there have been promising results using adoptive transfer of activated immune cells in infections with Candida spp., Aspergillus spp. and Mucorales in animal models129. Another fascinating example of work in immune cell antifungals is in targeting JUN amino-terminal kinase 1 (JNK1; also known as MAPK8), which has an important role in T cell activation and T helper cell differentiation. JNK1 negatively regulates the host antifungal innate immune response, and JNK1 inhibitors exert potent antifungal therapeutic effects both in mice and in human cells130. Finally, unlike for viral and bacterial infections, currently there is no commercial fungal vaccine for humans. There is, however, a substantial infrastructure of work in this area for proof of principle131– 137. In fact, there is fundamental knowledge that vaccines can prevent fungal infections in model systems, but very little data on therapeutic fungal vaccines exist. Preliminary human fungal vaccine studies have been carried out in cocci dioidomycosis and candidiasis138,139. A Candida spp. vaccine (NovaDigm Therapeutics) has been used in phase I and phase II studies (NCT01447407 and NCT01926028). Understanding the potential of these vaccines will require clinical studies in carefully identified at-risk groups to assess outcomes and the development of a financial plan for their implementation. Prevention is an important goal in the management of fungal infections. As host immunity is crucial for the long-term management of fungal infections, protection given by vaccines and/or immune cell-targeted approaches should receive high priority in the antifungal pipeline.
Antifungal biological agents
Although there are few primary antifungal therapy recommendations for the use of biological agents in the Infectious Diseases Society of America guidelines3–7, the area has received some attention and could be further developed. The use of monoclonal antibodies to attack fungi88,140 or even carry potent antifungal material, such as radiation (radioimmunotherapy), to the fungus has been validated in animal models141 as a potential treatment strategy. However, it should be noted how carefully these antibodies need to be tested. For instance, when the efungumab formulation was changed to an efungumab-C28Y variant, it lost its anti-Candida spp. activity89. The adjuvant therapeutic use of cytokines has been explored, and these molecules could be useful142,143, but precise control of immune reconstitution might need some fine-tuning.
Summary
The current antifungal tools that are available to tackle the invasive fungal epidemic occurring in the clinics and hospitals have improved, but are still inadequate for use in all patient groups. In response to this need for more and better antifungal agents, this Review has focused on four areas that could bolster and refine the antifungal pipeline: basic pursuits to identify fungal pathways, targets and mechanisms of action that could lead to new antifungal inhibitors; antifungal compounds and immune strategies currently in development that could become new antifungal therapies; improved formulations of existing compounds; and the repurposing of drugs approved for other indications that have the potential to be antifungal agents.
Invasive fungal infections will not go away anytime soon. Therefore, we need to circumvent resistance to treatment by continued discovery and development of new antifungal agents and strategies. Hopefully, these new fungicidal agents will meet the management challenges of an enlarging population of susceptible ‘human Petri dishes’ as we attempt to manage serious underlying diseases.
Acknowledgments
The author has research support from Public Health Service Grants (AI73896, AI04533, AI93257).
Glossary
- Mycetomas
Chronic fungal infections of skin and soft tissue
- T2 MRI
(Magnetic resonance imaging). A testing system from T2 Biosystems that uses advances in nanotechnology and molecular biology to detect microorganisms directly from body fluids
- GAIN Act
The Generating Antibiotic Incentives Now (GAIN) Act by the US Federal Government is legislation that aims to stimulate antimicrobial discovery
- Orphan Drug Act
The Orphan Drug Act enables the US Food and Drug Administration (FDA) to designate a treatment as being developed for a rare disease upon request from a sponsor
- Fast Track
Fast Track is a US Food and Drug Administration (FDA) designation for expedited review of drugs to fill unmet medical needs, as requested by a drug company
- Nosocomial
Hospital-based
- Virulence composite
The genetic and phenotypic traits that encompass pathogenicity
Footnotes
Competing interests statement
The author declares competing interests: see Web version for details.
DATABASES
ClinicalTrials.gov: https://clinicaltrials.gov/
FURTHER INFORMATION
Cloudbreak technology: https://www.cidara.com/cloudbreak/
MethylGene reports results of phase II trial of MGCD290: http://www.advfn.com/news_MethylGene-Reports-Results-of-Phase-II-Trial-of-MG_56785808.html
Researchers identify new class of antifungal agents: http://www.asm.org/index.php/journal-press-releases/93560-reserachers-identify-new-class-of-antifungal-agents
(SUBA)-itraconazole: https://www.maynepharma.com/
T-2307 developmental pipeline: http://www.toyama-chemical.co.jp/en/rd/pipeline/index.html
Viamet receives Orphan Drug Designation from the FDA for VT-1598 for the treatment of coccidioidomycosis: http://www.businesswire.com/news/home/20160525005794/en/Viamet-recives-orphen-drug-designation-FDA-VT-1598.2016
References
- 1.Brown GD, Denning DW, Levitz SM. Tackling human fungal infections. Science. 2012;336:647. doi: 10.1126/science.1222236. This work reviews the current magnitude of issues relating to invasive fungal infections. [DOI] [PubMed] [Google Scholar]
- 2.Park BJ, et al. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 3.Patterson TF, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63:e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pappas PG, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galgiani JN, et al. 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clin Infect Dis. 2016;63:e112–e146. doi: 10.1093/cid/ciw360. [DOI] [PubMed] [Google Scholar]
- 6.Perfect JR, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kauffman CA, Hajjeh R, Chapman SW. Practice guidelines for the management of patients with sporotrichosis. For the Mycoses Study Group. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:684–687. doi: 10.1086/313751. [DOI] [PubMed] [Google Scholar]
- 8.Oliver JD, et al. F901318 represents a novel class of antifungal drug that inhibits dihydroorotate dehydrogenase. Proc Natl Acad Sci USA. 2016;113:12809–12814. doi: 10.1073/pnas.1608304113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shahid SK. Newer patents in antimycotic therapy. Pharm Pat Anal. 2016;5:115–134. doi: 10.4155/ppa-2015-0001. [DOI] [PubMed] [Google Scholar]
- 10.Pouliot M, Jeanmart S. Pan assay interference compounds (PAINS) and other promiscuous compounds in antifungal research. J Med Chem. 2016;59:497–503. doi: 10.1021/acs.jmedchem.5b00361. [DOI] [PubMed] [Google Scholar]
- 11.Pitman SK, Drew RH, Perfect JR. Addressing current medical needs in invasive fungal infection prevention and treatment with new antifungal agents, strategies and formulations. Expert Opin Emerg Drugs. 2011;16:559–586. doi: 10.1517/14728214.2011.607811. This study is an extensive review of the available antifungal agents and discusses how they are optimally used today. [DOI] [PubMed] [Google Scholar]
- 12.Cuenca-Estrella M. Antifungal drug resistance mechanisms in pathogenic fungi: from bench to bedside. Clin Microbiol Infect. 2014;20(Suppl 6):54–59. doi: 10.1111/1469-0691.12495. [DOI] [PubMed] [Google Scholar]
- 13.Smith KD, et al. Increased antifungal drug resistance in clinical isolates of Cryptococcus neoformans in Uganda. Antimicrob Agents Chemother. 2015;59:7197–7204. doi: 10.1128/AAC.01299-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denning DW, Bromley MJ. Infectious disease. How to bolster the antifungal pipeline. Science. 2015;347:1414–1416. doi: 10.1126/science.aaa6097. This insightful discussion describes the potential needs and barriers to antifungal development. [DOI] [PubMed] [Google Scholar]
- 15.Ostrosky-Zeichner L, Casadevall A, Galgiani JN, Odds FC, Rex JH. An insight into the antifungal pipeline: selected new molecules and beyond. Nat Rev Drug Discov. 2010;9:719–727. doi: 10.1038/nrd3074. By comparing reference 15 and this Review, the progress that has been made in the antifungal pipeline in the past decade can be determined. [DOI] [PubMed] [Google Scholar]
- 16.Osherov N, Kontoyiannis DP. The anti-Aspergillus drug pipeline: is the glass half full or empty? Med Mycol. 2017;55:118–124. doi: 10.1093/mmy/myw060. [DOI] [PubMed] [Google Scholar]
- 17.Odds FC, Brown AJ, Gow NA. Antifungal agents: mechanisms of action. Trends Microbiol. 2003;11:272–279. doi: 10.1016/s0966-842x(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 18.Allen D, Wilson D, Drew R, Perfect J. Azole antifungals: 35 years of invasive fungal infection management. Expert Rev Anti Infect Ther. 2015;13:787–798. doi: 10.1586/14787210.2015.1032939. [DOI] [PubMed] [Google Scholar]
- 19.Hamill RJ. Amphotericin B formulations: a comparative review of efficacy and toxicity. Drugs. 2013;73:919–934. doi: 10.1007/s40265-013-0069-4. [DOI] [PubMed] [Google Scholar]
- 20.Holt SL, Drew RH. Echinocandins: addressing outstanding questions surrounding treatment of invasive fungal infections. Am J Health Syst Pharm. 2011;68:1207–1220. doi: 10.2146/ajhp100456. [DOI] [PubMed] [Google Scholar]
- 21.Peyton LR, Gallagher S, Hashemzadeh M. Triazole antifungals: a review. Drugs Today (Barc) 2015;51:705–718. doi: 10.1358/dot.2015.51.12.2421058. [DOI] [PubMed] [Google Scholar]
- 22.Nett JE, Andes DR. Antifungal agents: spectrum of activity, pharmacology, and clinical indications. Infect Dis Clin North Am. 2016;30:51–83. doi: 10.1016/j.idc.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Andes DR, et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis. 2012;54:1110–1122. doi: 10.1093/cid/cis021. [DOI] [PubMed] [Google Scholar]
- 24.Bratton EW, et al. Comparison and temporal trends of three groups with cryptococcosis: HIV-infected, solid organ transplant, and HIV-negative/non-transplant. PLoS ONE. 2012;7:e43582. doi: 10.1371/journal.pone.0043582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nyazika TK, et al. Cryptococcus neoformans population diversity and clinical outcomes of HIV-associated cryptococcal meningitis patients in Zimbabwe. J Med Microbiol. 2016;65:1281–1288. doi: 10.1099/jmm.0.000354. [DOI] [PubMed] [Google Scholar]
- 26.Marr KA, et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med. 2015;162:81–89. doi: 10.7326/M13-2508. [DOI] [PubMed] [Google Scholar]
- 27.Perfect JR. Fungal virulence genes as targets for antifungal chemotherapy. Antimicrob Agents Chemother. 1996;40:1577–1583. doi: 10.1128/aac.40.7.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitamura A, Someya K, Hata M, Nakajima R, Takemura M. Discovery of a small-molecule inhibitor of β-1,6-glucan synthesis. Antimicrob Agents Chemother. 2009;53:670–677. doi: 10.1128/AAC.00844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baxter BK, DiDone L, Ogu D, Schor S, Krysan DJ. Identification, in vitro activity and mode of action of phosphoinositide-dependent-1 kinase inhibitors as antifungal molecules. ACS Chem Biol. 2011;6:502–510. doi: 10.1021/cb100399x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breger J, et al. Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog. 2007;3:e18. doi: 10.1371/journal.ppat.0030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spitzer M, et al. Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Mol Syst Biol. 2011;7:499. doi: 10.1038/msb.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roemer T, et al. Confronting the challenges of natural product-based antifungal discovery. Chem Biol. 2011;18:148–164. doi: 10.1016/j.chembiol.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Tebbets B, et al. Identification and characterization of antifungal compounds using a Saccharomyces cerevisiae reporter bioassay. PLoS ONE. 2012;7:e36021. doi: 10.1371/journal.pone.0036021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krysan DJ, Didone L. A high-throughput screening assay for small molecules that disrupt yeast cell integrity. J Biomol Screen. 2008;13:657–664. doi: 10.1177/1087057108320713. [DOI] [PubMed] [Google Scholar]
- 35.Lamoth F, Juvvadi PR, Steinbach WJ. Editorial: advances in Aspergillus fumigatus pathobiology. Front Microbiol. 2016;7:43. doi: 10.3389/fmicb.2016.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juvvadi PR, Lee SC, Heitman J, Steinbach WJ. Calcineurin in fungal virulence and drug resistance: prospects for harnessing targeted inhibition of calcineurin for an antifungal therapeutic approach. Virulence. 2017;8:186–197. doi: 10.1080/21505594.2016.1201250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blankenship JR, Steinbach WJ, Perfect JR, Heitman J. Teaching old drugs new tricks: reincarnation immunosuppressants as antifungal drugs. Curr Opin Investig Drugs. 2003;4:192–199. [PubMed] [Google Scholar]
- 38.Cowen LE, et al. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc Natl Acad Sci USA. 2009;106:2818–2823. doi: 10.1073/pnas.0813394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pachl J, et al. A randomized, blinded, multicenter trial of lipid-associated Amphotericin B alone versus in combination with an antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin Infect Dis. 2006;42:1404–1413. doi: 10.1086/503428. [DOI] [PubMed] [Google Scholar]
- 40.Nambu M, et al. A calcineurin antifungal strategy with analogs of FK506. Bioorg Med Chem Lett. 2011 doi: 10.1016/j.bmcl.2017.04.004. http://dx.doi.org/10.1016/j.bmcl.2017.04.004. [DOI] [PubMed]
- 41.Hast MA, et al. Structures of Cryptococcus neoformans protein farnesyltransferase reveal strategies for developing inhibitors that target fungal pathogens. J Biol Chem. 2011;286:35149–35162. doi: 10.1074/jbc.M111.250506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mor V, et al. Identification of a new class of antifungals targeting the synthesis of fungal sphingolipids. mBio. 2015;6:e00647. doi: 10.1128/mBio.00647-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rollin-Pinheiro R, Singh A, Barreto-Bergter E, Del Poeta M. Sphingolipids as targets for treatment of fungal infections. Future Med Chem. 2016;8:1469–1484. doi: 10.4155/fmc-2016-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perfect JR, Tenor JL, Miao Y, Brennan RG. Trehalose pathway as an antifungal target. Virulence. 2016;8:143–149. doi: 10.1080/21505594.2016.1195529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petzold EW, et al. Characterization and regulation of the trehalose synthesis pathway and its importance in the pathogenicity of Cryptococcus neoformans. Infect Immun. 2006;74:5877–5887. doi: 10.1128/IAI.00624-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ngamskulrungroj P, et al. The trehalose pathway: an integral part of virulence composite for Cryptococcus gattii. Infect Immun. 2009;77:4584–4596. doi: 10.1128/IAI.00565-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miao Y, et al. Structures of trehalose-6-phosphate phosphatase from pathogenic fungi reveal the mechanisms of substrate recognition and catalysis. Proc Natl Acad Sci USA. 2016;113:7148–7153. doi: 10.1073/pnas.1601774113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheah HL, Lim V, Sandai D. Inhibitors of the glyoxylate cycle enzyme ICL1 in Candida albicans for potential use as antifungal agents. PLoS ONE. 2014;9:e95951. doi: 10.1371/journal.pone.0095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knauth P, Reichenbach H. On the mechanism of action of the myxobacterial fungicide ambruticin. J Antibiot (Tokyo) 2000;53:1182–1190. doi: 10.7164/antibiotics.53.1182. [DOI] [PubMed] [Google Scholar]
- 50.Levine HB, Ringel SM, Cobb JM. Therapeutic properties of oral ambruticin (W7783) in experimental pulmonary coccidioidomycosis of mice. Chest. 1978;73:202–206. doi: 10.1378/chest.73.2.202. [DOI] [PubMed] [Google Scholar]
- 51.Shubitz LF, et al. Efficacy of ambruticin analogs in a murine model of coccidioidomycosis. Antimicrob Agents Chemother. 2006;50:3467–3469. doi: 10.1128/AAC.00670-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hagihara K, et al. Fingolimod (FTY720) stimulates Ca2+/calcineurin signaling in fission yeast. PLoS ONE. 2013;8:e81907. doi: 10.1371/journal.pone.0081907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gallo-Ebert C, et al. Novel antifungal drug discovery based on targeting pathways regulating the fungus-conserved Upc2 transcription factor. Antimicrob Agents Chemother. 2014;58:258–266. doi: 10.1128/AAC.01677-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hein KZ, et al. Disulphide-reduced psoriasin is a human apoptosis-inducing broad-spectrum fungicide. Proc Natl Acad Sci USA. 2015;112:13039–13044. doi: 10.1073/pnas.1511197112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valiante V, et al. Hitting the caspofungin salvage pathway of human-pathogenic fungi with the novel lasso peptide humidimycin (MDN-0010) Antimicrob Agents Chemother. 2015;59:5145–5153. doi: 10.1128/AAC.00683-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu N, Wang C, Su H, Zhang W, Sheng C. Strategies in the discovery of novel antifungal scaffolds. Future Med Chem. 2016;8:1435–1454. doi: 10.4155/fmc-2016-0020. [DOI] [PubMed] [Google Scholar]
- 57.Scorzoni L, et al. Searching new antifungals: the use of in vitro and in vivo methods for evaluation of natural compounds. J Microbiol Methods. 2016;123:68–78. doi: 10.1016/j.mimet.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Nobrega RO, Teixeira AP, Oliveira WA, Lima EO, Lima IO. Investigation of the antifungal activity of carvacrol against strains of Cryptococcus neoformans. Pharm Biol. 2016;54:2591–2596. doi: 10.3109/13880209.2016.1172319. [DOI] [PubMed] [Google Scholar]
- 59.Zuo R, et al. In vitro antifungal and antibiofilm activities of halogenated quinoline analogues against Candida albicans and Cryptococcus neoformans. Int J Antimicrob Agents. 2016;48:208–211. doi: 10.1016/j.ijantimicag.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 60.Ghannoum MA, Kim HG, Long L. Efficacy of aminocandin in the treatment of immunocompetent mice with haematogenously disseminated fluconazole-resistant candidiasis. J Antimicrob Chemother. 2007;59:556–559. doi: 10.1093/jac/dkl525. [DOI] [PubMed] [Google Scholar]
- 61.Warn PA, Sharp A, Morrissey G, Denning DW. Activity of aminocandin (IP960; HMR3270) compared with amphotericin B, itraconazole, caspofungin and micafungin in neutropenic murine models of disseminated infection caused by itraconazole-susceptible and -resistant strains of Aspergillus fumigatus. Int J Antimicrob Agents. 2010;35:146–151. doi: 10.1016/j.ijantimicag.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 62.Hanadate T, et al. FR290581, a novel sordarin derivative: synthesis and antifungal activity. Bioorg Med Chem Lett. 2009;19:1465–1468. doi: 10.1016/j.bmcl.2009.01.051. [DOI] [PubMed] [Google Scholar]
- 63.Hasenoehrl A, et al. In vitro activity and in vivo efficacy of icofungipen (PLD-118), a novel oral antifungal agent, against the pathogenic yeast Candida albicans. Antimicrob Agents Chemother. 2006;50:3011–3018. doi: 10.1128/AAC.00254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sigurgeirsson B, van Rossem K, Malahias S, Raterink K. A phase II, randomized, double-blind, placebo-controlled, parallel group, dose-ranging study to investigate the efficacy and safety of 4 dose regimens of oral albaconazole in patients with distal subungual onychomycosis. J Am Acad Dermatol. 2013;69:416–425. doi: 10.1016/j.jaad.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 65.Miller JL, et al. In vitro and in vivo efficacies of the new triazole albaconazole against Cryptococcus neoformans. Antimicrob Agents Chemother. 2004;48:384–387. doi: 10.1128/AAC.48.2.384-387.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakamura I, et al. ASP2397: a novel antifungal agent produced by Acremonium persicinum MF-347833. J Antibiot (Tokyo) 2017;70:45–51. doi: 10.1038/ja.2016.107. [DOI] [PubMed] [Google Scholar]
- 67.Nakamura I, et al. Discovery of a new antifungal agent ASP2397 using a silkworm model of Aspergillus fumigatus infection. J Antibiot (Tokyo) 2017;70:41–44. doi: 10.1038/ja.2016.106. [DOI] [PubMed] [Google Scholar]
- 68.Nishikawa H, et al. T-2307, a novel arylamidine, is transported into Candida albicans by a high-affinity spermine and spermidine carrier regulated by Agp2. J Antimicrob Chemother. 2016;71:1845–1855. doi: 10.1093/jac/dkw095. [DOI] [PubMed] [Google Scholar]
- 69.Shibata T, et al. T-2307 causes collapse of mitochondrial membrane potential in yeast. Antimicrob Agents Chemother. 2012;56:5892–5897. doi: 10.1128/AAC.05954-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mitsuyama J, et al. In vitro and in vivo antifungal activities of T-2307, a novel arylamidine. Antimicrob Agents Chemother. 2008;52:1318–1324. doi: 10.1128/AAC.01159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamada E, Nishikawa H, Nomura N, Mitsuyama J. T-2307 shows efficacy in a murine model of Candida glabrata infection despite in vitro trailing growth phenomena. Antimicrob Agents Chemother. 2010;54:3630–3634. doi: 10.1128/AAC.00355-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koselny K, et al. The celecoxib derivative AR-12 has broad spectrum antifungal activity in vitro and improves the activity of fluconazole in a murine model of cryptococcosis. Antimicrob Agents Chemother. 2016;60:7115–7127. doi: 10.1128/AAC.01061-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koselny K, et al. Antitumor/antifungal celecoxib derivative AR-12 is a non-nucleoside inhibitor of the ANL-family adenylating enzyme acetyl CoA synthetase. ACS Infect Dis. 2016;2:268–280. doi: 10.1021/acsinfecdis.5b00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richard ML, Plaine A. Comprehensive analysis of glycosylphosphatidylinositol-anchored proteins in Candida albicans. Eukaryot Cell. 2007;6:119–133. doi: 10.1128/EC.00297-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Watanabe NA, et al. E1210, a new broad-spectrum antifungal, suppresses Candida albicans hyphal growth through inhibition of glycosylphosphatidylinositol biosynthesis. Antimicrob Agents Chemother. 2012;56:960–971. doi: 10.1128/AAC.00731-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McLellan CA, et al. Inhibiting GPI anchor biosynthesis in fungi stresses the endoplasmic reticulum and enhances immunogenicity. ACS Chem Biol. 2012;7:1520–1528. doi: 10.1021/cb300235m. [DOI] [PubMed] [Google Scholar]
- 77.Hata K, et al. Efficacy of oral E1210, a new broad-spectrum antifungal with a novel mechanism of action, in murine models of candidiasis, aspergillosis, and fusariosis. Antimicrob Agents Chemother. 2011;55:4543–4551. doi: 10.1128/AAC.00366-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wiederhold NP, et al. The investigational agent E1210 is effective in treatment of experimental invasive candidiasis caused by resistant Candida albicans. Antimicrob Agents Chemother. 2015;59:690–692. doi: 10.1128/AAC.03944-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miyazaki M, et al. In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrob Agents Chemother. 2011;55:4652–4658. doi: 10.1128/AAC.00291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shubitz LF, et al. Modeling nikkomycin Z dosing and pharmacology in murine pulmonary coccidioidomycosis preparatory to phase 2 clinical trials. J Infect Dis. 2014;209:1949–1954. doi: 10.1093/infdis/jiu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hector RF, Zimmer BL, Pappagianis D. Evaluation of nikkomycins X and Z in murine models of coccidioidomycosis, histoplasmosis, and blastomycosis. Antimicrob Agents Chemother. 1990;34:587–593. doi: 10.1128/aac.34.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fortwendel JR, et al. Differential effects of inhibiting chitin and 1,3-β-D-glucan synthesis in ras and calcineurin mutants of Aspergillus fumigatus. Antimicrob Agents Chemother. 2009;53:476–482. doi: 10.1128/AAC.01154-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lilla EA, Yokoyama K. Carbon extension in peptidylnucleoside biosynthesis by radical SAM enzymes. Nat Chem Biol. 2016;12:905–907. doi: 10.1038/nchembio.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pfaller MA, et al. Activity of MGCD290, a Hos2 histone deacetylase inhibitor, in combination with azole antifungals against opportunistic fungal pathogens. J Clin Microbiol. 2009;47:3797–3804. doi: 10.1128/JCM.00618-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pfaller MA, Rhomberg PR, Messer SA, Castanheira M. In vitro activity of a Hos2 deacetylase inhibitor, MGCD290, in combination with echinocandins against echinocandin-resistant Candida species. Diagn Microbiol Infect Dis. 2015;81:259–263. doi: 10.1016/j.diagmicrobio.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 86.Cowen LE. The fungal Achilles’ heel: targeting Hsp90 to cripple fungal pathogens. Curr Opin Microbiol. 2013;16:377–384. doi: 10.1016/j.mib.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 87.Lamoth F, Alexander BD, Juvvadi PR, Steinbach WJ. Antifungal activity of compounds targeting the Hsp90-calcineurin pathway against various mould species. J Antimicrob Chemother. 2015;70:1408–1411. doi: 10.1093/jac/dku549. [DOI] [PubMed] [Google Scholar]
- 88.Bugli F, et al. Human monoclonal antibody-based therapy in the treatment of invasive candidiasis. Clin Dev Immunol. 2013;2013:403121. doi: 10.1155/2013/403121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Louie A, et al. Dose range evaluation of Mycograb C28Y variant, a human recombinant antibody fragment to heat shock protein 90, in combination with amphotericin B-desoxycholate for treatment of murine systemic candidiasis. Antimicrob Agents Chemother. 2011;55:3295–3304. doi: 10.1128/AAC.01324-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aeed PA, Young CL, Nagiec MM, Elhammer AP. Inhibition of inositol phosphorylceramide synthase by the cyclic peptide aureobasidin A. Antimicrob Agents Chemother. 2009;53:496–504. doi: 10.1128/AAC.00633-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kurome T, Inoue T, Takesako K, Kato I. Syntheses of antifungal aureobasidin A analogs with alkyl chains for structure-activity relationship. J Antibiot. 1998;51:359–367. doi: 10.7164/antibiotics.51.359. [DOI] [PubMed] [Google Scholar]
- 92.Kumaresan PR, et al. Bioengineering T cells to target carbohydrate to treat opportunistic fungal infection. Proc Natl Acad Sci USA. 2014;111:10660–10665. doi: 10.1073/pnas.1312789111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arikan S, Rex JH. Nystatin LF (Aronex/Abbott) Curr Opin Investig Drugs. 2001;2:488–495. [PubMed] [Google Scholar]
- 94.Janout V, Bienvenu C, Schell W, Perfect JR, Regen SL. Molecular umbrella-amphotericin B conjugates. Bioconjug Chem. 2014;25:1408–1411. doi: 10.1021/bc500277v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang Z, et al. Development and characterization of amphotericin B nanosuspensions for oral administration through a simple top-down method. Curr Pharm Biotechnol. 2014;15:569–576. doi: 10.2174/1389201015666140706160709. [DOI] [PubMed] [Google Scholar]
- 96.Halperin A, et al. Novel water-soluble amphotericin B-PEG conjugates with low toxicity and potent in vivo efficacy. J Med Chem. 2016;59:1197–1206. doi: 10.1021/acs.jmedchem.5b01862. [DOI] [PubMed] [Google Scholar]
- 97.Ickowicz DE, et al. Activity, reduced toxicity, and scale-up synthesis of amphotericin B-conjugated polysaccharide. Biomacromolecules. 2014;15:2079–2089. doi: 10.1021/bm5002125. [DOI] [PubMed] [Google Scholar]
- 98.Jung SH, et al. Amphotericin B-entrapping lipid nanoparticles and their in vitro and in vivo characteristics. Eur J Pharm Sci. 2009;37:313–320. doi: 10.1016/j.ejps.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 99.Janout V, et al. Taming amphotericin B. Bioconjug Chem. 2015;26:2021–2024. doi: 10.1021/acs.bioconjchem.5b00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Delmas G, et al. Efficacy of orally delivered cochleates containing amphotericin B in a murine model of aspergillosis. Antimicrob Agents Chemother. 2002;46:2704–2707. doi: 10.1128/AAC.46.8.2704-2707.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ong V, et al. Preclinical evaluation of the stability, safety and efficacy of CD101, a novel echinocandin. Antimicrob Agents Chemother. 2016;60:6872–6879. doi: 10.1128/AAC.00701-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pfaller MA, Messer SA, Rhomberg PR, Jones RN, Castanheira M. Activity of a longacting echinocandin, CD101, determined using CLSI and EUCAST reference methods, against Candida and Aspergillus spp., including echinocandin- and azole-resistant isolates. J Antimicrob Chemother. 2016;71:2868–2873. doi: 10.1093/jac/dkw214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Walker SS, et al. Discovery of a novel class of orally active antifungal β-1,3-D-glucan synthase inhibitors. Antimicrob Agents Chemother. 2011;55:5099–5106. doi: 10.1128/AAC.00432-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gebremariam T, et al. VT-1161 protects immunosuppressed mice from Rhizopus arrhizus var. arrhizus infection. Antimicrob Agents Chemother. 2015;59:7815–7817. doi: 10.1128/AAC.01437-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Warrilow AG, et al. The clinical candidate VT-1161 is a highly potent inhibitor of Candida albicans CYP51 but fails to bind the human enzyme. Antimicrob Agents Chemother. 2014;58:7121–7127. doi: 10.1128/AAC.03707-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gupta AK, Studholme C. Novel investigational therapies for onychomycosis: an update. Expert Opin Investig Drugs. 2016;25:297–305. doi: 10.1517/13543784.2016.1142529. [DOI] [PubMed] [Google Scholar]
- 107.Lockhart SR, et al. The investigational fungal Cyp51 inhibitor VT-1129 demonstrates potent in vitro activity against Cryptococcus neoformans and Cryptococcus gattii. Antimicrob Agents Chemother. 2016;60:2528–2531. doi: 10.1128/AAC.02770-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Butts A, Krysan DJ. Antifungal drug discovery: something old and something new. PLoS Pathog. 2012;8:e1002870. doi: 10.1371/journal.ppat.1002870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Christenson JC, Shalit I, Welch DF, Guruswamy A, Marks MI. Synergistic action of amphotericin B and rifampin against Rhizopus species. Antimicrob Agents Chemother. 1987;31:1775–1778. doi: 10.1128/aac.31.11.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu S, et al. Synergistic effect of fluconazole and calcium channel blockers against resistant Candida albicans. PLoS ONE. 2016;11:e0150859. doi: 10.1371/journal.pone.0150859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Coelho C, Casadevall A. Cryptococcal therapies and drug targets: the old, the new and the promising. Cell Microbiol. 2016;18:792–799. doi: 10.1111/cmi.12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shirazi F, Kontoyiannis DP. The calcineurin pathway inhibitor tacrolimus enhances the in vitro activity of azoles against Mucorales via apoptosis. Eukaryot Cell. 2013;12:1225–1234. doi: 10.1128/EC.00138-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Husain S, Wagner M, Singh N. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg Infect Dis. 2001;7:375–381. doi: 10.3201/eid0703.010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Duke SS, Fromtling RA. Effects of diethylstilbestrol and cyclophosphamide on the pathogenesis of experimental Cryptococcus neoformans infections. Sabouraudia. 1984;22:125–135. [PubMed] [Google Scholar]
- 115.Mohr JA, Tatem BA, Long H, Muchmore HG, Felton FG. Increased susceptibility of Cryptococcus neoformans to amphotericin B in the presence of steroids. Sabouraudia. 1973;11:140–142. [PubMed] [Google Scholar]
- 116.Butts A, et al. Estrogen receptor antagonists are anti-cryptococcal agents that directly bind EF hand proteins and synergize with fluconazole in vivo. mBio. 2014;5:e00765–13. doi: 10.1128/mBio.00765-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dolan K, et al. Antifungal activity of tamoxifen: in vitro and in vivo activities and mechanistic characterization. Antimicrob Agents Chemother. 2009;53:3337–3346. doi: 10.1128/AAC.01564-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhai B, Wu C, Wang L, Sachs MS, Lin X. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob Agents Chemother. 2012;56:3758–3766. doi: 10.1128/AAC.00212-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rhein J, et al. Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: an open-label dose-ranging study. Lancet Infect Dis. 2016;16:809–818. doi: 10.1016/S1473-3099(16)00074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Del Poeta M, et al. Comparison of in vitro activities of camptothecin and nitidine derivatives against fungal and cancer cells. Antimicrob Agents Chemother. 1999;43:2862–2868. doi: 10.1128/aac.43.12.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cardenas ME, et al. Antifungal activities of antineoplastic agents: Saccharomyces cerevisiae as a model system to study drug action. Clin Microbiol Rev. 1999;12:583–611. doi: 10.1128/cmr.12.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Widmer F, et al. Hexadecylphosphocholine (miltefosine) has broad-spectrum fungicidal activity and is efficacious in a mouse model of cryptococcosis. Antimicrob Agents Chemother. 2006;50:414–421. doi: 10.1128/AAC.50.2.414-421.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wiederhold NP, et al. Limited activity of miltefosine in murine models of cryptococcal meningoencephalitis and disseminated cryptococcosis. Antimicrob Agents Chemother. 2013;57:745–750. doi: 10.1128/AAC.01624-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pace JR, et al. Repurposing the clinically efficacious antifungal agent itraconazole as an anticancer chemo therapeutic. J Med Chem. 2016;59:3635–3649. doi: 10.1021/acs.jmedchem.5b01718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Abuhelwa AY, Foster DJ, Mudge S, Hayes D, Upton RN. Population pharmacokinetic modeling of itraconazole and hydroxyitraconazole for oral SUBA-itraconazole and sporanox capsule formulations in healthy subjects in fed and fasted states. Antimicrob Agents Chemother. 2015;59:5681–5696. doi: 10.1128/AAC.00973-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Le J, Schiller DS. Aerosolized delivery of antifungal agents. Curr Fungal Infect Rep. 2010;4:96–102. doi: 10.1007/s12281-010-0011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pornputtapitak W, El-Gendy N, Berkland C. NanoCluster itraconazole formulations provide a potential engineered drug particle approach to generate effective dry powder aerosols. J Aerosol Med Pulm Drug Deliv. 2015;28:341–352. doi: 10.1089/jamp.2014.1155. [DOI] [PubMed] [Google Scholar]
- 128.Walraven CJ, Lee SA. Antifungal lock therapy. Antimicrob Agents Chemother. 2013;57:1–8. doi: 10.1128/AAC.01351-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tramsen L, et al. Clinical-scale generation of multi-specific anti-fungal T cells targeting Candida, Aspergillus and mucormycetes. Cytotherapy. 2013;15:344–351. doi: 10.1016/j.jcyt.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 130.Zhao X, et al. JNK1 negatively controls antifungal innate immunity by suppressing CD23 expression. Nat Med. 2017;23:337–346. doi: 10.1038/nm.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Galgiani JN. Vaccines to prevent systemic mycoses: holy grails meet translational realities. J Infect Dis. 2008;197:938–940. doi: 10.1086/529205. This article discusses the potential for antifungal vaccines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Casadevall A, Pirofski LA. Feasibility and prospects for a vaccine to prevent cryptococcosis. Med Mycol. 2005;43:667–680. doi: 10.1080/13693780500448230. [DOI] [PubMed] [Google Scholar]
- 133.Cutler JE, Deepe GS, Jr, Klein BS. Advances in combating fungal diseases: vaccines on the threshold. Nat Rev Microbiol. 2007;5:13–28. doi: 10.1038/nrmicro1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Levitz SM. Aspergillus vaccines: hardly worth studying or worthy of hard study? Med Mycol. 2017;55:103–108. doi: 10.1093/mmy/myw081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Upadhya R, et al. Induction of protective immunity to cryptococcal infection in mice by a heat-killed, chitosan-deficient strain of Cryptococcus neoformans. mBio. 2016;7:e00547–16. doi: 10.1128/mBio.00547-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mor V, et al. Glucosylceramide administration as a vaccination strategy in mouse models of cryptococcosis. PLoS ONE. 2016;11:e0153853. doi: 10.1371/journal.pone.0153853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wormley FL, Jr, Perfect JR, Steele C, Cox GM. Protection against cryptococcosis by using a murine gamma interferon-producing Cryptococcus neoformans strain. Infect Immun. 2007;75:1453–1462. doi: 10.1128/IAI.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pappagianis D. Evaluation of the protective efficacy of the killed Coccidioides immitis spherule vaccine in humans. The Valley Fever Vaccine Study Group. Am Rev Respir Dis. 1993;148:656–660. doi: 10.1164/ajrccm/148.3.656. [DOI] [PubMed] [Google Scholar]
- 139.Edwards JE., Jr Fungal cell wall vaccines: an update. J Med Microbiol. 2012;61:895–903. doi: 10.1099/jmm.0.041665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Larsen RA, et al. Phase I evaluation of the safety and pharmacokinetics of murine-derived anticryptococcal antibody 18B7 in subjects with treated cryptococcal meningitis. Antimicrob Agents Chemother. 2005;49:952–958. doi: 10.1128/AAC.49.3.952-958.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bryan RA, et al. Toward developing a universal treatment for fungal disease using radioimmunotherapy targeting common fungal antigens. Mycopathologia. 2012;173:463–471. doi: 10.1007/s11046-011-9476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jarvis J, et al. Adjunctive interferon-γ immnotherapy for the treatment of HIV-associated cryptococcal meningitis: a randomized controlled trial. AIDS. 2012;26:1105–1113. doi: 10.1097/QAD.0b013e3283536a93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pappas PG, et al. Recombinant interferon-γ 1b as adjunctive therapy for AIDS-related acute cryptococcal meningitis. J Infect Dis. 2004;189:2185–2191. doi: 10.1086/420829. [DOI] [PubMed] [Google Scholar]
- 144.Rodero L, et al. In vitro susceptibility studies of Cryptococcus neoformans isolated from patients with no clinical response to amphotericin B therapy. J Antimicrob Chemother. 2000;45:239–242. doi: 10.1093/jac/45.2.239. [DOI] [PubMed] [Google Scholar]
- 145.Sanglard D, Odds FC. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect Dis. 2002;2:73–85. doi: 10.1016/s1473-3099(02)00181-0. [DOI] [PubMed] [Google Scholar]
- 146.Verweij PE, Chowdhary A, Melchers WJ, Meis JF. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis. 2016;62:362–368. doi: 10.1093/cid/civ885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Alexander BD, et al. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis. 2013;56:1724–1732. doi: 10.1093/cid/cit136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Healey KR, et al. Prevalent mutator genotype identified in fungal pathogen Candida glabrata promotes multidrug resistance. Nat Commun. 2016;7:11128. doi: 10.1038/ncomms11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Edlind TD, Katiyar SK. Mutational analysis of flucytosine resistance in Candida glabrata. Antimicrob Agents Chemother. 2010;54:4733–4738. doi: 10.1128/AAC.00605-10. [DOI] [PMC free article] [PubMed] [Google Scholar]