Abstract
Background & objectives:
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease which affects females more than males. Gender affects the manifestations of SLE and men with lupus show more severe symptoms and worse prognosis. This study was aimed to compare clinical and immunological features in female and male lupus patients in Iran.
Methods:
Demographic, clinical and laboratory data from 78 women and 20 men with lupus were collected. Autoantibodies (against nRNP, Sm, SSA, SSB, Ro-52, CENP, Jo-1, Scl-70, nucleosome, anti-dsDNA, histone and Rib-p protein) were determined using immunoblotting technique.
Results:
Men with lupus had less anti-SSA (21.1 vs 48.1%) and anti-Ro52 (10.5 vs 44.3%) antibodies when compared to women and none of the male patients had anti-SSB antibodies. Kidney damage was more frequent in men (68.4% in men vs 36.7% in women). In men with kidney involvement, anti-dsDNA increased significantly (84.6 vs 20.0%) in comparison to males without nephritis. Anti-SSA (7.7 vs 50.0%) and anti-nRNP (0.0 vs 33.8%) on the other hand, decreased. Women with renal involvement had no anti-SSB antibodies.
Interpretation & conclusions:
In male patients, SLE appeared with more severe features, and kidney damage was more frequent in males. The frequency of some autoantibodies was different between females and males. In males with kidney damage anti-dsDNA increased significantly, while anti-SSA and anti-nRNP decreased. Anti-SSB was not detected in males and females with nephritis.
Keywords: ANA, ANA profile, anti-DNA, autoantibody, gender, systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disease characterized by the presence of autoantibodies directed against nuclear antigens1. Autoantibodies either directly and/ or by immune complex formation mediate inflammation and damage of diverse organs2. SLE is a disorder with a variety of clinical manifestations, and a profound sex bias3, which mostly affects females more than males, but men with lupus show more severe symptoms4,5, and worse prognosis6,7. Although sex hormones are considered to be essential factors for the development of clinical differences and gender bias in SLE8,9,10,11, genetic, immunologic, hormonal and environmental factors could also affect the clinical features, disease outcomes and severity of SLE12,13,14.
Females and males show distinct immune characteristics, and it is considered that female sex hormones cause enhanced immune responses3. In previous studies, different aspects of lupus in both sexes in different geographical regions were studied15,16. The prevalence of SLE in Iran has been reported to be 40/100,00017, and more severe symptoms have been observed in Iranian lupus patients compared to European Caucasians18. As ethnic, racial and socio-economic factors are implicated in the development of clinical symptoms and severity of lupus in females and males19,20 the current study was aimed to determine the clinical and immunological features in male and female SLE patients in Iran.
Material & Methods
The study was conducted in the Immunology Research Centre (Ghaem hospital, Mashhad, Iran) between 2011 and 2013. All consecutive lupus patients fulfilling the 1997 American College of Rheumatology (ACR)21 revised criteria centre were included in the study.
Inclusion criteria included the following: (i) Patients who were newly diagnosed (before starting treatment); (ii) Patients in remission who took only a maximum dose of 10 mg/day prednisolone and/or 200 mg/day of hydroxychloroquine; and (iii) Patients with active major organ involvement were sampled (5 ml) before starting cytotoxic or a high dose of corticosteroid therapy (sampling was performed before changing treatment strategy). Some of them were inpatients. Patients with drug-induced lupus and with overlap syndromes were excluded.
The patients were evaluated for demographic, clinical and laboratory data. Clinical variables were obtained using history, and physical examination and disease activity for each patient was determined using Systemic Lupus Erythematosus Disease Activity Index (SLEDAI)22. Organ involvement was also defined according to SLEDAI criteria. Autoantibodies against nRNP, Sm, SSA, SSB, Ro-52, CENP B, Jo-1, Scl-70, nucleosomes, anti-dsDNA, histones and Rib-p protein were determined by a commercial kit (EUROIMMUN; Germany) using immunoblotting technique according to the manufacturer's instructions. Briefly, 1.5 ml of each diluted serum was incubated with a strip of pre-coated antigens (nRNP, Sm, SSA, SSB, Ro-52, CENP B, Jo-1, Scl-70, nucleosomes, anti-dsDNA, histones and Rib-p protein) for 30 min at room temperature. Strips were then washed three times using washing buffer, and incubated with 1.5 ml of diluted enzyme conjugated anti-human IgG for 30 min. After washing, strips were incubated with 1.5 ml of substrate solution for 10 min, then washed, air dried and evaluated for the presence of autoantibodies.
The study protocol was approved by the ethics committee of Mashhad University of Medical Sciences, Iran, and written informed consent was obtained from all patients.
Statistical analysis: Statistical analysis was performed using SPSS 16.0 for windows (Chicago, IL, USA). Comparison between categorical variables was made using Chi-square or if necessary, Fisher's exact test. Comparison between continuous variables was made using Student's t test or Mann–Whitney test. To confirm statistical analysis on individual level multivariate analyses using binary logistic regression or exact logistic regression was carried out using STATA statistical software (release 11.1, 2009; Stata Corporation, USA). Effects on risk were estimated by odds ratio with 95 per cent confidence intervals (CI). All analyses were adjusted for patient's age at the sampling time.
Results
Of the 98 SLE patients 78 were women with a mean age of 27.5±7.9 yr and 20 were men with mean age of 27.0±8.0 years. Disease duration in male SLE patients who were in remission was 4.99±4.1 yr (range 0.5-15 yr) and in females it was 4.31±3.5 yr (range 0.3-17 yr). In the current study, 41.8 per cent of patients (n=41) were from rural areas and 58.2 per cent (n=57) were from urban areas.
Clinical manifestations in male and female SLE patients: There was no difference between women and men in the total number of involved organs (3.0±1.1 for women vs 2.7±1.0 for men). In men, kidney damage and in women, arthritis were the most frequent clinical disorders. The increased frequency of kidney damage (OR=0.31, 95% CI=0.11-0.87) and oral ulcer (OR=0.06, 95% CI=0.006-0.7) in men were significant compared to women. Data on clinical variables are shown in Table I.
Table I.
Clinical and demographic variables in female (n=78) and male (n=20) systemic lupus erythematosus (SLE) patients
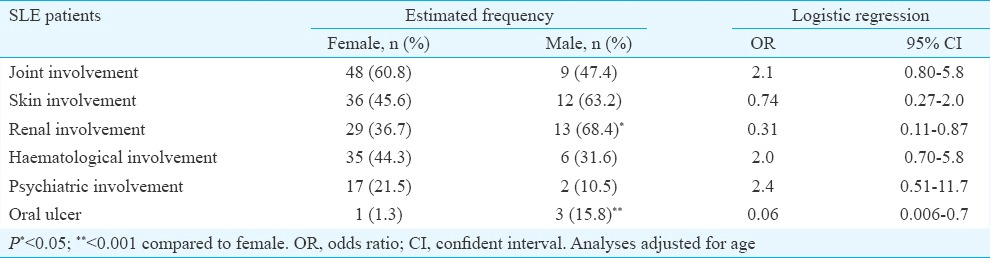
In those patients with lupus nephritis (n=42), 73.8 per cent (n=31) had proteinuria (more than 0.5 g/day), 26.2 per cent (n=11) had urinary cellular casts, and in 42.9 per cent cases (n=18) nephritis was confirmed with biopsy as well. The patients with urinary cellular cast were included in the study after biopsy confirmation or concomitant proteinuria.
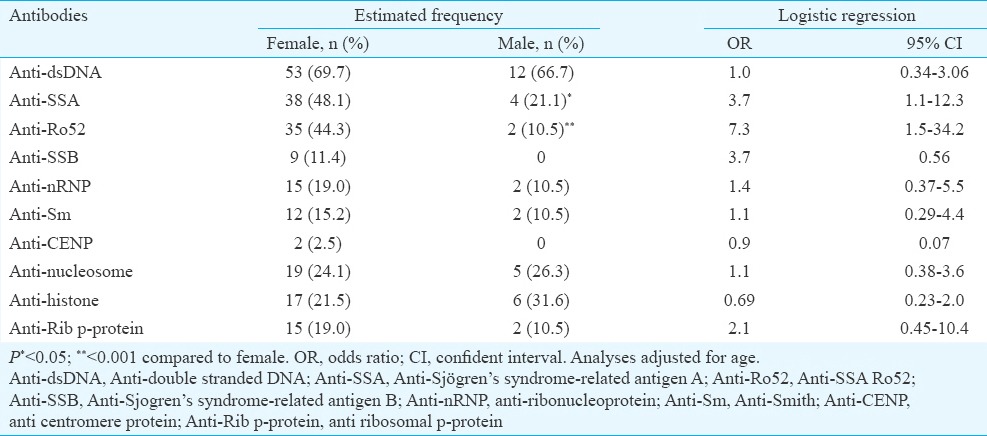
Frequency of autoantibodies in male and female SLE patients: In lupus patients at the sampling time, there existed various autoantibodies while anti-dsDNA was the most frequent antibody in both genders. In male SLE patients, anti-SSA (OR=3.7, 95% CI=1.1-12.3) and anti-Ro52 (OR=7.3, 95% CI=1.5-34.2) autoantibodies were significantly less frequent than females, and none of the men with SLE had anti-SSB (Table II).
Table II.
Frequency of autoantibodies in female (n=78) and male (n=20) systemic lupus erythematosus (SLE) patients
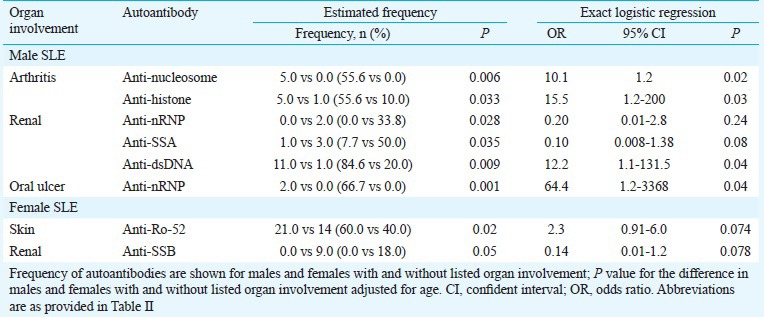
Comparison of autoantibodies in patients with and without renal involvement: In men SLE patients with renal involvement, the frequency of anti-dsDNA increased significantly in comparison to males without it (P =0.009). Decreased frequency of anti-SSA and anti-SSB antibodies was significant in male patients with renal damage (P =0.035; P =0.028, respectively).
None of the female patients with kidney involvement had anti-SSB autoantibodies (OR=0.14, 95% CI=0.01-1.2). Table III represents the data for significant autoantibodies in patients with and without renal involvement.
Table III.
Significant autoantibodies in each affected organ in male (n=20) and female (n=78) systemic lupus erythematosus (SLE) patients
Discussion
In the present study, the difference of clinical manifestations and immunological factors were analysed in female and male SLE patients with a comparable mean age. Laboratory findings and clinical manifestations of SLE are highly affected by ethnicity, genetics, race and environmental factors14. Immunological data of our study were comparable to others23 and showed that anti-dsDNA was the most common autoantibody in both sexes, while anti-SSA and anti-Ro52 autoantibodies decreased in male patients in comparison to females, and none of males had anti-SSB. Decreased frequency of anti-SSA and anti-SSB in male SLE was previously reported in some studies4,24.
In our study, kidney damage was more frequent in male patients. Severe renal damage24,25,26, and inferior outcome of the disease in male SLE patients was reported in the previous studies4,24. The frequency of anti-dsDNA was increased in both sexes, but, in male SLE, kidney damage was more common than females. It is well established that not all patients with anti-dsDNA develop nephritis, and also not all anti-dsDNA antibodies are pathogenic. The ability of anti-dsDNA antibodies to induce renal damage depends on the properties of antibody such as its affinity, isotype, charge, cross reactivity and also its capability for binding to a kidney antigen27,28.
It was found that in male patients with nephritis, in addition to the significant increase in the frequency of anti-dsDNA, there existed a synchronized decrease in the frequency of anti-SSA, anti-nRNP and a lack of anti-SSB autoantibodies. An association between the presence of anti-nRNP and lower risk of renal diseases has been reported29. Migliorini et al30 showed an association of anti-nRNP with milder renal disease and others reported that positive anti-SSB was associated with decreased likelihood and severity of renal disease31,32. In our study also, anti-SSA and anti-nRNP decreased in males with nephritis, while anti-SSB was absent in both female and male patients with renal damages. The possible mechanism of this effect remains elusive and requires more studies. A protective role for anti-SSA, anti-SSB and anti-nRNP against renal injuries has been suggested in the previous studies29,33,34.
This study had some limitations, as it was a cross-sectional study with a small sample size; moreover, as this study was conducted in a tertiary centre it may has some selection bias. Therefore, to generalize such results, it needs a longitudinal study with higher number of patients.
In conclusion, the results of this study showed that male patients with SLE were more likely to develop kidney damage. In men with nephritis, the frequency of anti-dsDNA increased significantly, while anti-SSA, anti-SSB and anti-nRNP decreased. Increased frequency of anti-dsDNA with a decrease in other antibodies may affect the outcome of the SLE in male patients. Further studies with a large sample need to be done to understand the mechanism.
Acknowledgment
The authors acknowledge the Vice President of Research of Mashhad University of Medical Sciences, Iran, for providing financial support.
Footnotes
Conflicts of Interest: None.
References
- 1.Wu Y, Cai B, Feng W, Yang B, Huang Z, Zuo C, et al. Double positive CD4+CD8+ T cells: Key suppressive role in the production of autoantibodies in systemic lupus erythematosus. Indian J Med Res. 2014;140:513–9. [PMC free article] [PubMed] [Google Scholar]
- 2.Yap DY, Lai KN. Pathogenesis of renal disease in systemic lupus erythematosus - the role of autoantibodies and lymphocytes subset abnormalities. Int J Mol Sci. 2015;16:7917–31. doi: 10.3390/ijms16047917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy G, Isenberg D. Effect of gender on clinical presentation in systemic lupus erythematosus. Rheumatology (Oxford) 2013;52:2108–15. doi: 10.1093/rheumatology/ket160. [DOI] [PubMed] [Google Scholar]
- 4.Tan TC, Fang H, Magder LS, Petri MA. Differences between male and female systemic lupus erythematosus in a multiethnic population. J Rheumatol. 2012;39:759–69. doi: 10.3899/jrheum.111061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soto ME, Vallejo M, Guillén F, Simón JA, Arena E, Reyes PA. Gender impact in systemic lupus erythematosus. Clin Exp Rheumatol. 2004;22:713–21. [PubMed] [Google Scholar]
- 6.Gómez J, Suárez A, López P, Mozo L, Díaz JB, Gutiérrez C. Systemic lupus erythematosus in Asturias, Spain: Clinical and serologic features. Medicine (Baltimore) 2006;85:157–68. doi: 10.1097/01.md.0000224711.54886.b1. [DOI] [PubMed] [Google Scholar]
- 7.Font J, Cervera R, Navarro M, Pallarés L, López-Soto A, Vivancos J, et al. Systemic lupus erythematosus in men: Clinical and immunological characteristics. Ann Rheum Dis. 1992;51:1050–2. doi: 10.1136/ard.51.9.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hochberg MC. Systemic lupus erythematosus. Rheum Dis Clin North Am. 1990;16:617–39. [PubMed] [Google Scholar]
- 9.Cohen-Solal JF, Jeganathan V, Grimaldi CM, Peeva E, Diamond B. Sex hormones and SLE: Influencing the fate of autoreactive B cells. Curr Top Microbiol Immunol. 2006;305:67–88. doi: 10.1007/3-540-29714-6_4. [DOI] [PubMed] [Google Scholar]
- 10.Rastin M, Hatef MR, Tabasi N, Mahmoudi M. The pathway of estradiol-induced apoptosis in patients with systemic lupus erythematosus. Clin Rheumatol. 2012;31:417–24. doi: 10.1007/s10067-011-1821-3. [DOI] [PubMed] [Google Scholar]
- 11.Rider V, Abdou NI. Gender differences in autoimmunity: Molecular basis for estrogen effects in systemic lupus erythematosus. Int Immunopharmacol. 2001;1:1009–24. doi: 10.1016/s1567-5769(01)00046-7. [DOI] [PubMed] [Google Scholar]
- 12.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 13.Rhodes B, Vyse TJ. The genetics of SLE: An update in the light of genome-wide association studies. Rheumatology (Oxford) 2008;47:1603–11. doi: 10.1093/rheumatology/ken247. [DOI] [PubMed] [Google Scholar]
- 14.Sestak AL, Fürnrohr BG, Harley JB, Merrill JT, Namjou B. The genetics of systemic lupus erythematosus and implications for targeted therapy. Ann Rheum Dis. 2011;70(Suppl 1):i37–43. doi: 10.1136/ard.2010.138057. [DOI] [PubMed] [Google Scholar]
- 15.Kamen DL, Barron M, Parker TM, Shaftman SR, Bruner GR, Aberle T, et al. Autoantibody prevalence and lupus characteristics in a unique African American population. Arthritis Rheum. 2008;58:1237–47. doi: 10.1002/art.23416. [DOI] [PubMed] [Google Scholar]
- 16.Carbone LD, Lohr KM. Ethnic differences in male lupus. J Clin Rheumatol. 2002;8:239–40. doi: 10.1097/00124743-200208000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Davatchi F, Jamshidi AR, Banihashemi AT, Gholami J, Forouzanfar MH, Akhlaghi M, et al. WHO-ILAR COPCORD Study (Stage 1, Urban Study) in Iran. J Rheumatol. 2008;35:1384. [PubMed] [Google Scholar]
- 18.Akbarian M, Faezi ST, Gharibdoost F, Shahram F, Nadji A, Jamshidi AR, et al. Systemic lupus erythematosus in Iran: A study of 2280 patients over 33 years. Int J Rheum Dis. 2010;13:374–9. doi: 10.1111/j.1756-185X.2010.01547.x. [DOI] [PubMed] [Google Scholar]
- 19.Alarcón GS, Roseman JM, McGwin G, Jr, Uribe A, Bastian HM, Fessler BJ, et al. Systemic lupus erythematosus in three ethnic groups. XX. Damage as a predictor of further damage. Rheumatology (Oxford) 2004;43:202–5. doi: 10.1093/rheumatology/keg481. [DOI] [PubMed] [Google Scholar]
- 20.Ong C, Nicholls K, Becker G. Ethnicity and lupus nephritis: An Australian single centre study. Intern Med J. 2011;41:270–8. doi: 10.1111/j.1445-5994.2009.02159.x. [DOI] [PubMed] [Google Scholar]
- 21.Hochberg M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 22.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 23.Pradhan V, Patwardhan M, Nadkarni A, Ghosh K. Fcγ R IIB gene polymorphisms in Indian systemic lupus erythematosus (SLE) patients. Indian J Med Res. 2011;134:181–5. [PMC free article] [PubMed] [Google Scholar]
- 24.Feng JB, Ni JD, Yao X, Pan HF, Li XP, Xu JH, et al. Gender and age influence on clinical and laboratory features in Chinese patients with systemic lupus erythematosus: 1,790 cases. Rheumatol Int. 2010;30:1017–23. doi: 10.1007/s00296-009-1087-0. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman IE, Peene I, Meheus L, Huizinga TW, Cebecauer L, Isenberg D, et al. Specific antinuclear antibodies are associated with clinical features in systemic lupus erythematosus. Ann Rheum Dis. 2004;63:1155–8. doi: 10.1136/ard.2003.013417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rivest C, Lew RA, Welsing PM, Sangha O, Wright EA, Roberts WN, et al. Association between clinical factors, socioeconomic status, and organ damage in recent onset systemic lupus erythematosus. J Rheumatol. 2000;27:680–4. [PubMed] [Google Scholar]
- 27.Smeenk RJ, Van Rooijen A, Swaak TJ. Dissociation studies of DNA/anti-DNA complexes in relation to anti-DNA avidity. J Immunol Methods. 1988;109:27–35. doi: 10.1016/0022-1759(88)90438-3. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Z, Weinstein E, Tuzova M, Davidson A, Mundel P, Marambio P, et al. Cross-reactivity of human lupus anti-DNA antibodies with alpha-actinin and nephritogenic potential. Arthritis Rheum. 2005;52:522–30. doi: 10.1002/art.20862. [DOI] [PubMed] [Google Scholar]
- 29.Reichlin M, Van Venrooij WJ. Autoantibodies to the URNP particles: Relationship to clinical diagnosis and nephritis. Clin Exp Immunol. 1991;83:286–90. doi: 10.1111/j.1365-2249.1991.tb05629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Migliorini P, Baldini C, Rocchi V, Bombardieri S. Anti-Sm and anti-RNP antibodies. Autoimmunity. 2005;38:47–54. doi: 10.1080/08916930400022715. [DOI] [PubMed] [Google Scholar]
- 31.Castro C, Gourley M. Diagnostic testing and interpretation of tests for autoimmunity. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S238–47. doi: 10.1016/j.jaci.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherer Y, Gorstein A, Fritzler MJ, Shoenfeld Y. Autoantibody explosion in systemic lupus erythematosus: More than 100 different antibodies found in SLE patients. Semin Arthritis Rheum. 2004;34:501–37. doi: 10.1016/j.semarthrit.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Tápanes FJ, Vásquez M, Ramírez R, Matheus C, Rodríguez MA, Bianco N. Cluster analysis of antinuclear autoantibodies in the prognosis of SLE nephropathy: Are anti-extractable nuclear antibodies protective? Lupus. 2000;9:437–44. doi: 10.1191/096120300678828604. [DOI] [PubMed] [Google Scholar]
- 34.Malik S, Bruner GR, Williams-Weese C, Feo L, Scofield RH, Reichlin M, et al. Presence of anti-La autoantibody is associated with a lower risk of nephritis and seizures in lupus patients. Lupus. 2007;16:863–6. doi: 10.1177/0961203307083365. [DOI] [PubMed] [Google Scholar]


