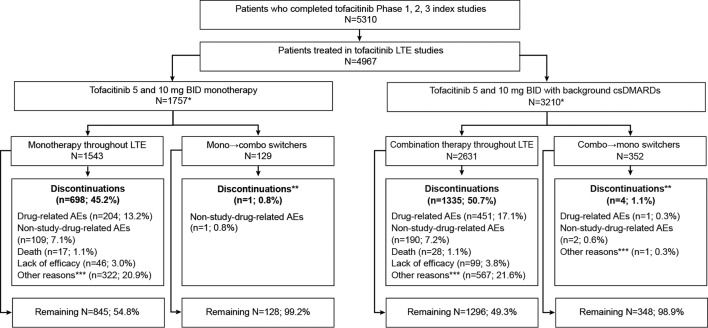
Figure 1.
Patient disposition. *Patients who switched multiple times were not included in this analysis; **for patients switching treatment regimens, discontinuations from study within 30 days of treatment switch are reported; ***‘other reasons’ included patients lost to follow-up, patients no longer willing to participate, withdrawals due to pregnancy, protocol violations and study termination by sponsor. AE, adverse event; BID, twice daily; csDMARD, conventional synthetic disease-modifying antirheumatic drug; LTE, long-term extension.