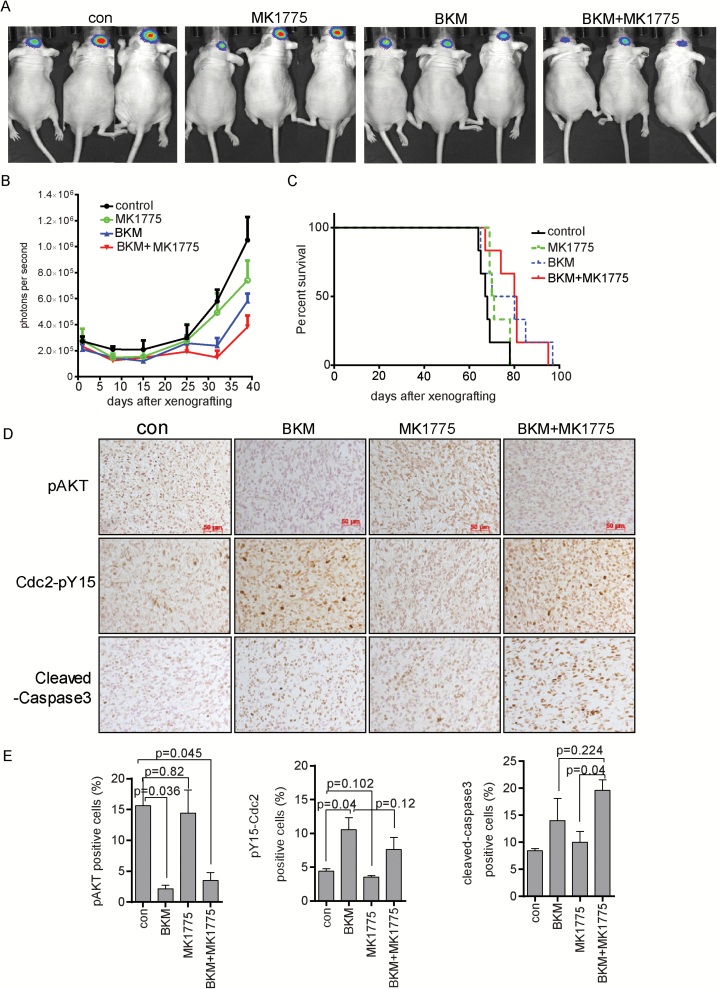
Fig. 6.
Evaluation of efficacy of MK1775 and BKM in an intracranial animal model. (A) Mice were administered BKM (20 mg/kg/d), MK1775 (30 mg/kg/d), or combination by gavage 5 times a week for 5 weeks. Representative bioluminescence imaging of tumor at day 39 is shown. (B) Quantitative assessments of tumor growth following implantation. Data expressed as mean + SD, n = 6 per group. P = 0.208 for control vs MK1775; P = 0.028 for control vs BKM; P = 0.0169 for control vs combination; P = 0.079 for MK1775 vs combination; P = 0.38 for BKM vs combination. (C) Mice were sacrificed at morbidity, survival curves were plotted in Kaplan–Meier graphs, and differences evaluated using the log-rank test (P = 0.0126 for control vs combination; P = 0.045 for MK1775 vs combination; P = 0.978 for BKM vs combination). (D) Immunostaining of the brain sections of animals that had been treated for 5 weeks. The tissue sections were incubated with antibodies against pS473-Akt, pY15-Cdc2, and cleaved caspase-3. Scale bars, 50 microns.