Analyses deriving insights from 23 research sites across the northeastern and east central US reveals that white-tailed deer lower native plant diversity and increase the fraction of the plant community made up of non-native species. Particularly, deer increase the abundance of the invasive plants garlic mustard and Japanese stiltgrass. Deer are known to avoid eating these species in favour of more palatable ones, thereby indirectly facilitating the success of the non-native invasives. Managing deer abundance has implications for forest plant communities. By maintaining lower deer densities, native plants likely will be better sustained and invasions of unpalatable non-native plants limited.
Keywords: Biological invasions, exotic plants, herbivory, introduced plants, Odocoileus virginianus, palatability, plant invasion, regional pooled analysis
Abstract
Herbivores can profoundly influence plant species assembly, including plant invasion, and resulting community composition. Population increases of native herbivores, e.g. white-tailed deer (Odocoileus virginianus), combined with burgeoning plant invasions raise concerns for native plant diversity and forest regeneration. While individual researchers typically test for the impact of deer on plant invasion at a few sites, the overarching influence of deer on plant invasion across regional scales is unclear. We tested the effects of deer on the abundance and diversity of introduced and native herbaceous and woody plants across 23 white-tailed deer research sites distributed across the east-central and north-eastern USA and representing a wide range of deer densities and invasive plant abundance and identity. Deer access/exclusion or deer population density did not affect introduced plant richness or community-level abundance. Native and total plant species richness, abundance (cover and stem density) and Shannon diversity were lower in deer-access vs. deer-exclusion plots. Among deer-access plots, native species richness, native and total cover, and Shannon diversity (cover) declined as deer density increased. Deer access increased the proportion of introduced species cover (but not of species richness or stem density). As deer density increased, the proportion of introduced species richness, cover and stem density all increased. Because absolute abundance of introduced plants was unaffected by deer, the increase in proportion of introduced plant abundance is likely an indirect effect of deer reducing native cover. Indicator species analysis revealed that deer access favoured three introduced plant species, including Alliaria petiolata and Microstegium vimineum, as well as four native plant species. In contrast, deer exclusion favoured three introduced plant species, including Lonicera japonica and Rosa multiflora, and 15 native plant species. Overall, native deer reduced community diversity, lowering native plant richness and abundance, and benefited certain invasive plants, suggesting pervasive impacts of this keystone herbivore on plant community composition and ecosystem services in native forests across broad swathes of the eastern USA.
Introduction
Modern plant communities are anthropogenically altered (Hannah et al. 1994). Habitat loss and forest fragmentation have contributed to acute reductions in biodiversity, species homogenization, and concomitant proliferation of invasive species and some large herbivores (McKinney and Lockwood 1999; Rooney et al. 2004; Alroy 2008). Because large mammalian herbivores can play a prominent role in determining plant community composition (Harper 1977; Crawley 1997; Russell et al. 2001; Côté et al. 2004), understanding their effects on plant species and communities, including plant invasions, is critical for conserving biodiversity.
Large herbivores affect plant communities directly via tissue loss and plant mortality, indirectly through non-consumptive effects including trampling (Persson et al. 2000; Heckel et al. 2010), accelerating nutrient cycling (Hobbs 1996; Rooney and Waller 2003) and by dispersing plant propagules (Vellend 2002; Myers et al. 2004; Bartuszevige and Endress 2008; Williams et al. 2008; Castellano and Gorchov 2013). Perhaps the most pervasive effect of large mammals on plant communities, however, is their indirect impact of altering interspecific plant competition through selective herbivory and plant response to herbivory (Holt 1977; Bowers 1993; Crawley 1997; Augustine and McNaughton 1998), with large impacts on community assembly and succession (Drake 1990; Hobbs 1996). For example, herbivores can alter successional trajectories when they preferentially consume early or late successional plant species (Hobbs 1996; Crawley 1997; Côté et al. 2004; DiTommaso et al. 2014; Forsyth et al. 2015). Consumption of palatable species can cause unpalatable species to gain an apparent competitive advantage and potentially become dominant or invasive (Leopold et al. 1947; Holt 1977; Augustine and McNaughton 1998; Horsley et al. 2003; Côté et al. 2004; Vavra et al. 2007). For example, pastures and rangeland can become infested with Carduus, Centaurea and Cirsium spp., among others, when grazers consume more palatable species (DiTomaso 2000). Selective herbivory can result in woody plant invasion in savannas, i.e. encroachment, which occurs as grazers reduce herbaceous species, indirectly facilitating establishment of unpalatable woody vegetation (Asner et al. 2004), but the more common result is a reduction of palatable woody plants, which slows succession from field to forest (DiTommaso et al. 2014; Habeck and Schultz 2015). The selective browsing of cervids (e.g. deer, moose, elk) is considered one of the main determinants of forest understory plant species composition and structure (Alverson et al. 1988; Côté et al. 2004; Abrams 2013). Herbivore-mediated shifts in plant communities can limit native plant regeneration, alter the abundance of small mammals, birds and insects, lower ecological stability (e.g. erosion and flood protection), disrupt ecosystem functioning, induce alternative stable states, reduce the economic value of habitats (reviewed in Côté et al. 2004) and trigger or facilitate plant invasions (Stromayer and Warren 1997; Vavra et al. 2007).
In North America, many large native herbivores, including bison (Bison bison), caribou (Rangifer tarandus), Dall’s sheep (Ovis dalli), elk (Cervus elaphus), moose (Alces alces) and pronghorn (Antilocapra americana), have experienced severe range contractions during the past 200 years (Laliberte and Ripple 2004). However, the range and abundance of native white-tailed deer (Odocoileus virginianus; hereafter referred to as deer) increased steadily following steep population declines in the late 1800s (McCabe and McCabe 1997; Laliberte and Ripple 2004). Low predator populations (Laliberte and Ripple 2004) and game laws that restricted hunting, in addition to increasing agricultural, silvicultural and early successional habitat, enhanced deer habitat within the past century, resulting in high deer populations (Alverson et al. 1988; McShea et al. 1997; Waller and Alverson 1997; Côté et al. 2004). Today, deer are the dominant wild ruminant herbivore in east-central and north-eastern USA and, because of their high abundance, are a serious ecological and management concern (McShea et al. 1997; Rooney 2001; McShea 2012). While deer at low abundances can increase floristic diversity (Royo et al. 2010a; Cook-Patton et al. 2014), abundant deer limit diversity and promote floral homogeneity (Rooney et al. 2004; Wiegmann and Waller 2006). At chronically high densities, deer change plant community structure and composition enough to be considered ‘ecosystem engineers’ or ‘keystone herbivores’ (Alverson et al. 1988; Waller and Alverson 1997; Côté et al. 2004). In many areas, deer population densities greatly exceed ecosystem carrying capacity (Rooney and Waller 2003), causing long-lasting and potentially irreversible legacy effects (Royo et al. 2010b; Nuttle et al. 2011).
As deer abundance increased during the past century, so did abundance of introduced plants, resulting in often concurrent ecological impacts. Human transport facilitates movement of many species outside their native ranges and, consequently, non-native species are now prominent components of present-day communities (Lockwood et al. 2013). Introduced plant species pose a growing threat to native plant communities, as their presence is associated with altered diversity, community structure and ecosystem function (MacDougall and Turkington 2005; Ehrenfeld 2010; Vilà et al. 2011; Beasley and McCarthy 2011). The fact that populations of deer and introduced plants have expanded concurrently suggests that deer abundance might be linked to introduced plant invasions (Augustine and McNaughton 1998; Vavra et al. 2007). However, data are lacking on regional effects of deer on native plant communities and plant invasion (Maron and Vilà 2001; Russell et al. 2001; Mosbacher and Williams 2009).
Throughout the past century, numerous experiments using fenced (deer-exclusion) and unfenced (deer-access) plots gauged deer impacts on forest plant communities (e.g. see McShea et al. 1997; Côté et al. 2004; Abrams and Johnson 2012; Habeck and Schultz 2015). Use of paired plots affords valuable insight into effects of large herbivores on floristic composition and on native and introduced plants, yet site-level studies assessing the degree to which deer influence introduced plants have yielded equivocal results. Several paired-plot experiments report deer facilitate certain invasive plants (Knight et al. 2009; Eschtruth and Battles 2009b; Beasley and McCarthy 2011; Kalisz et al. 2014; Dávalos et al. 2015b), others report deer mitigate invasions of different species (Rossell et al. 2007; Shelton et al. 2014) and others find no effect (Bowers 1993; Levine et al. 2012; DiTommaso et al. 2014) or mixed effects (Cadenasso et al. 2002; Webster et al. 2005; Knapp et al. 2008; Shen et al. 2016). Site-level investigations can provide practical insights about local species and conditions, but cannot be extrapolated to regional assessments about deer herbivory and plant invasion. A regional assessment requires data on a range of plant community types across a range of deer densities. Spatially broad investigations can bolster generalizations and forecasts made about ecological processes (Clark et al. 1999, 2001), such as community assembly and plant invasion (Gill and Beardall 2001; Shen et al. 2016).
Here, we present results of a multisite, regional assessment of white-tailed deer effects on composition, richness and abundance of introduced and native plants in east-central and north-eastern USA. We pool data from 23, paired-plot deer access/exclusion experiments spanning a broad range of invasive plant abundance and deer densities. We predicted that deer access would (i) alter floristic composition and reduce floristic diversity and (ii) increase richness and abundance of introduced plant species and decrease richness and abundance of native plant species.
Methods
Data description
We compiled data sets in which herbaceous and woody floristic composition and abundance were quantified in replicated deer-exclusion and deer-access plot experiments across 23 sites, resulting in 446 experimental units (223 plot pairs) (Table 1). We acquired data sets by directly contacting investigators of previously published (15 sites) and unpublished data (6 sites) and collecting additional data from established plots (2 sites, Long Run and Marienville) (Table 1). Sites were located in temperate deciduous or mixed deciduous forests across east-central and north-eastern USA (Table 1; Fig. 1). Sites were initially established to answer a range of research questions, not solely the effects of deer on introduced plants (Table 1). Overstory species typically included oak (Quercus spp.), maple (Acer spp.), beech (Fagus grandifolia), tulip-poplar (Liriodendron tulipifera) and black cherry (Prunus serotina) (Table 1). Deer density estimates varied across sites from 4 to 107 deer km−2 (Table 1; for estimation methods used, see Supporting Information—Table S1). The timing and duration of deer exclusion varied across experiments (Table 1). Six sites were established in the late 1980s/early 1990s, and the remaining 17 sites in the 2000s. At 15 sites, deer exclusion was imposed for 6 years or less, while at the other eight sites it ranged from 8 to 17 years. During the summer growing season, abundance data of herbaceous and woody species up to 2 m in height was recorded. Sampling intensity, plot area and replication varied across sites (Table 1). A recent meta-analysis showed no relationship between plot area and plant community responses to deer (Habeck and Schultz 2015). Fence heights used to exclude deer were a minimum of 2 m. Fence mesh size varied across experiments; therefore, deer may not have been the only mammalian herbivore excluded (e.g. see Bowers 1993).
Table 1.
Descriptions of 23 experimental sites and data used in pooled analyses testing the effect of white-tailed deer on introduced and native plants in east-central and north-eastern USA. Floristic composition data were collected from deer-access (unfenced) and Table 1A deer-exclusion (fenced) plots.
| Site (code) | US state | Latitude | Longitude | Dominant overstory species | Vegetation abundance measurement | Reference used for plant classification | Initial purpose/establishment of experiment | Estimated deer densityc | Duration of deer exclusion | Years of study | # Plot pairs | Plot aread | Subplot area | Total area sampled/plot | Distance between paired plots | Fence height | Fence mesh size | Data source; notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Decimal degrees | ||||||||||||||||||
| Antietam National Battlefield (AN) | MD | 39.4763 | −77.7490 | Maple, white ash, cherry | Density, cover classesa or density classes in subplots; sapling density in main plot | Strausbaugh and Core (1978); Brown and Brown (1984); Gleason and Cronquist (1991) | Woody seedling establishmentb | 53 | 6 | 2003–09 | 12 | 25 | 1 | 4 | <5 | 2.4 | 10 × 10 | McShea and Bourg (2009) |
| Catoctin Mountain Park (CA) | MD | 39.6561 | −77.4786 | Maple, tulip poplar | Density or cover classes | Newcomb (1977); USDA NRCS (2012) | Deer effects on plant composition in blow-down gaps created by hurricane Ivan | 44 | 3 | 2005–08 | 7 | 25 | None | 25 | 5 | 3 | 10 × 20 | Caraher (2009) |
| Chesapeake & Ohio Canal National Historical Park (CH) | MD | 39.0882 | −77.4619 | Maple, white ash, cherry | Density, cover classesa or density classes in subplots; sapling density in main plot | Strausbaugh and Core (1978); Brown and Brown (1984); Gleason and Cronquist (1991) | Woody seedling establishmentb | 54 | 6 | 2003–09 | 28 | 25 | 1 | 4 | <5 | 2.4 | 10 × 10 | McShea and Bourg (2009) |
| Smithsonian Conservation Biology Institute (CR) | VA | 38.8885 | −78.1434 | Oak, beech | Density, cover classesa or density classes | Strausbaugh and Core (1978); Brown and Brown (1984); Gleason and Cronquist (1991) | Deer and invasive plant interactions in upland forest | 107 | 4 | 2005–09 | 14 | 16 | 1 | 4 | 50 | 2.4 | 5 × 5 | Unpublished data, W. J. McShea and N. A. Bourg, SI Conservation Biology Institute |
| Smithsonian Environmental Research Center (SE) | MD | 38.8908 | −76.5646 | Tulip poplar, sweet gum, beech | Per cent cover | Gleason and Cronquist (1991); botanists (see note) | Deer effects on plant composition (random site selection) | 4 | 2 | 2009–11 | 16 | 100 | 1 | 5 | 3–10 | 2.3 | 50 × 50 | Unpublished data, J. D. Parker, SI; species ID: pers. comm. with botanists at SI Museum of Natural History |
| Fermilab (FE) | IL | 41.8423 | −88.2631 | Oak, ash, basswood | Cover classes | Swink and Wilhelm (1994) | Vegetation recovery after deer exclusion in two upland forests, one with historically rich flora | 6 | 14 | 1992–2006 | 3 | 594 | 1 | 25 | 5 | 3 | 15 × 15 | Unpublished data, V. Nuzzo, Natural Area Consultants; 90% deer herd cull in 1998 |
| Fernow (FN) | WV | 39.0167 | −79.7000 | Oak, maple, beech | Density and per cent cover | Gleason and Cronquist (1991); USDA NRCS (2012) | Disturbance and deer interactions | 6 | 6 | 2000–06 | 4 | 400 | 1 | 5 | >20 | 2 | 15 × 15 or 30 | Royo et al. (2010a) |
| Gold Mine Tract of C&O Canal (GM) | MD | 38.9931 | −77.2392 | Oak, beech | Density, cover classesa or density classes | Strausbaugh and Core (1978); Brown and Brown (1984); Gleason and Cronquist (1991) | Deer and invasive plant interactions in upland forest | 45 | 4 | 2005–09 | 10 | 16 | 1 | 4 | 50 | 2.4 | 5 × 5 | McShea and Bourg (2008) |
| Great Falls Park (GF) | VA | 38.9840 | −77.2531 | Oak, beech | Density, cover classesa or density classes | Strausbaugh and Core (1978); Brown and Brown (1984); Gleason and Cronquist (1991) | Deer and invasive plant interactions in upland forest | 26 | 4 | 2005–09 | 22 | 16 | 1 | 4 | 50 | 2.4 | 5 × 5 | McShea and Bourg (2008) |
| Long Run (LR) | PA | 41.6288 | −78.7211 | Black cherry, red maple | Per cent cover | Rhoads et al. (2007); USDA NRCS (2012); botanists (see note) | Deer and fern effects on woody seedling recruitment | 5 | 11 | 2000–11 | 5 | 280 | 1 | 4 | 10–30 | 2 | 5 × 5 | Unpublished data, K. M. Averill and D. A. Mortensen and A. A. Royo, USDA Forest Service; species ID: pers. comm. with botanists at Penn State |
| Manassas National Battlefield Park (MA) | VA | 38.8266 | −77.5279 | Oak, hickory, VA pine, northern red cedar | Density and per cent cover | Strausbaugh and Core (1978); Brown and Brown (1984); Gleason and Cronquist (1991) | Woody seedling establishmentb | 63 | 9 | 2000–09 | 23 | 12 | 1 | 4 | 1 | 2 | 5 × 10 | McShea et al. (2010) |
| Marienville (MV) | PA | 41.5347 | −79.1643 | Black cherry, red maple | Per cent cover | Rhoads et al. (2007); USDA NRCS (2012); botanists (see note) | Deer and fern effects on woody seedling recruitment | 5 | 11 | 2000–11 | 5 | 280 | 1 | 4 | 10–30 | 2 | 5 × 5 | Unpublished data, K. M. Averill and D. A. Mortensen and A. A. Royo, USDA Forest Service; species ID: pers. comm. with botanists at Penn State |
| Monocacy National Battlefield (MO) | MD | 39.3697 | −77.3924 | Dry oak, tulip poplar | Density or cover classesa in subplots; sapling density in main plot | Strausbaugh and Core (1978); Brown and Brown (1984); Gleason and Cronquist (1991) | Woody seedling establishmentb | 77 | 6 | 2003–09 | 6 | 25 | 1 | 4 | <5 | 2.4 | 10 × 10 | McShea and Bourg (2009) |
| Monongahela (MG) | WV | 39.1000 | −79.7167 | Oak, maple, beech | Density and per cent cover | Gleason and Cronquist (1991); USDA NRCS (2012) | Disturbance and deer interactions | 6 | 6 | 2000–06 | 4 | 400 | 1 | 5 | >20 | 2 | 15 × 15 or 30 | Royo et al. (2010a) |
| Morristown National Historic Park (MP) | NJ | 40.7760 | −74.5301 | Tulip poplar, white ash, oak, black locust | Cover classes | Newcomb (1977); Gleason and Cronquist (1991) | Plant composition and community structure | 19 | 14–17 | 1987–2005 | 5 | 36 | 1 | 9 | ~9 | 3.7 | 11 × 15 | Unpublished data, R. Masson, National Park Service |
| Raccoon Ecological Management Area (R1) | OH | 39.1997 | −82.4093 | Oak, hickory | Cover classes | Gleason and Cronquist (1991) | Acorns and oak regeneration (stratified random sampling) | 11 | 5 | 2001–06 | 3 | 400 | 1 | 12 | <5 | 2.4 | 4.4 × 5 | Unpublished data, T. Hutchinson and D. K. Apsley, USDA Forest Service |
| Riverbend Park (RB) | VA | 39.0145 | −77.2522 | Oak, beech | Density, cover classesa, or density classes | Strausbaugh and Core (1978); Brown and Brown (1984); Gleason and Cronquist (1991) | Deer and invasive plant interactions in upland forest | 26 | 3 | 2006–09 | 2 | 16 | 1 | 4 | 50 | 2.4 | 5 × 5 | McShea and Bourg (2008) |
| Shenandoah National Park (SH) | VA | 38.7438 | −78.2992 | Oak, hickory, pine | Density or density classes | Gleason and Cronquist (1991) | Acorn, rodent, bird interactions; deer and ecosystem interactions | 10 | 4–6 | 1990–96 | 6 | 4 ha | 1 | 18 | >1 km | 2.4 | 15 × 15 | McShea and Rappole (2000); McShea (2000); plots paired regionally, each with 3 24 × 24 m plots |
| Trillium Trail (TR) | PA | 40.5201 | −79.9011 | Oak, beech, maple, tulip poplar | Per cent cover | Gleason and Cronquist (1991) | Paired plots established to contain same native species with similar abiotic conditions | 32 | 8 | 1994–2002 | 3 | 100 | None | 100 | ~60 | 2.5 | 6 × 6 | Knight et al. (2009) |
| Valley Forge National Historical Park–Mt Joy (VJ) | PA | 40.0940 | −75.4543 | Tulip poplar, dry oak | Cover classes or density | Gleason and Cronquist (1991) | Plant composition; largest contiguous park woodlands selected | 84 | 17 | 1993–2010 | 15 | 9 | 4 | 4 | 20–36 | 2 | 5 × 10 | Abrams and Johnson (2012); 2 m metal stake in centres of control plots |
| Valley Forge National Historical Park–Mt Misery (VM) | PA | 40.0932 | −75.4611 | Dry oak | Cover classes or density | Gleason and Cronquist (1991) | Plant composition; largest contiguous park woodlands selected | 84 | 17 | 1993–2010 | 15 | 9 | 4 | 4 | 20–36 | 2 | 5 × 10 | Abrams and Johnson (2012); 2 m metal stake in centres of control plots |
| West Point (WP) | NY | 41.3636 | −74.0239 | Oak, sugar maple | Cover classes | Rhoads et al. (2007); USDA NRCS (2012) | Multiple stressor effects including deer and invasive plants; upland forests selected, half with invasive plants and half with none, without knowledge of deer abundance | No estimate available | 4 | 2008–12 | 12 | 900 | 1 | 10 | 5–50 | 2.4 | 5 × 5 | Nuzzo et al., this issue |
| Zaleski (Z1) | OH | 39.3032 | −82.3461 | Oak, hickory | Cover classes | Gleason and Cronquist (1991) | Acorns and oak regeneration | 11 | 5 | 2001–06 | 3 | 400 | 1 | 12 | <5 | 2.4 | 4.4 × 5 | Unpublished data, T. Hutchinson and D. K. Apsley, USDA Forest Service |
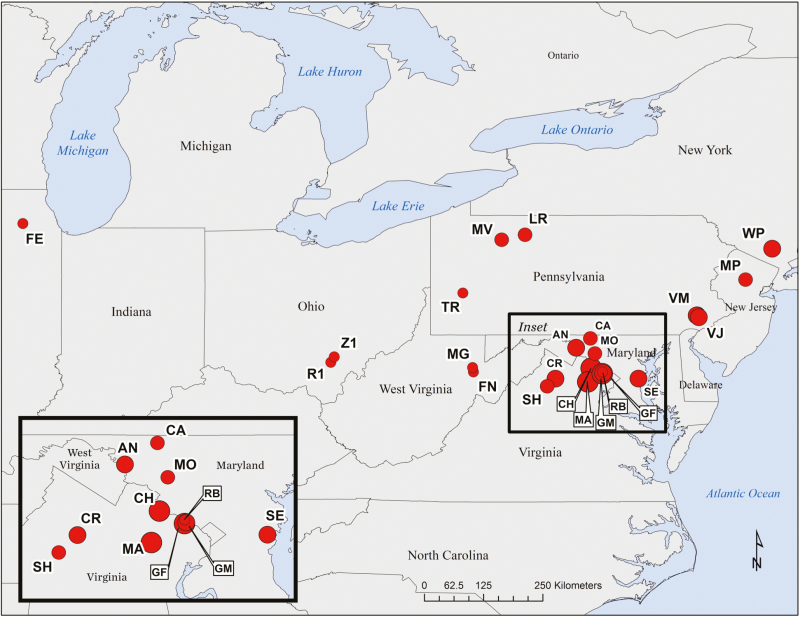
Figure 1.
Locations of 23 deer research sites in east-central and north-eastern USA included in pooled analyses. Symbol size indicates sampling intensity across sites, which are labelled with two-letter codes (see Table 1 for additional site information).
We acknowledge that the paired-plot approach has limitations, including fence-line effects, fences providing artificial support for vines and concentrated perch areas for birds, and an unrealistic total absence of deer in fenced plots (Russell et al. 2001; White 2012). Deer also presumably exerted an influence prior to experimentation (Russell et al. 2001), leaving behind legacy effects even after culling (Royo et al. 2010b; Nuttle et al. 2011), such as altered seedbank composition (DiTommaso et al. 2014), which could limit vegetation response to deer exclusion. Beyond the scope of this work, drivers of invasion could vary between areas with deer access vs. deer exclusion. Despite these limitations, herbivore-exclusion experiments remain among the most straightforward of ways to test the effects of herbivores on plant invasions (e.g. Parker et al. 2006).
Data set pooling
The pooling approach taken here has the benefit of increasing statistical power and reducing type II error rates (i.e. false negatives; Blettner et al. 1999). We processed the most recent floristic assessment from each experiment to analyse vegetation patterns at single points in time. However, we recognize that plant communities and deer densities vary temporally. Thus, analysing the temporal aspect of plant community assembly could improve conclusions about the interaction between deer and plant invasion since legacy effects play out over decadal time frames (Royo et al. 2010b; Nuttle et al. 2011; White 2012). Nonetheless, because sampling was spread across a wide range of sites and years, we expect observed patterns to be robust. We analysed equal numbers of deer-exclusion and deer-access plots from each data set (Table 1) and weighted plots equally.
We analysed plant species presence/absence and two abundance metrics, cover and stem density. Across experiments, plant abundance was quantified in several ways, including stem density (14 sites), per cent cover (8 sites), cover classes (i.e. ranges of per cent cover; 15 sites) and/or density classes (i.e. ranges of population density; 7 sites) (Table 1; for ranges of cover classes used and for treatment of density classes, see Supporting Information—Fig. S1, Text S1). We converted cover data to cover classes (for detailed processing methods, see Supporting Information—Text S1) and then used midpoints of cover classes (e.g. the midpoint of a 5–25 % cover class is 15 %) in analyses (hereafter referred to as cover).
Some plant species in almost every data set were unidentified and marked as unknown at genus, family or growth habit level (e.g. forb, fern, graminoid, woody seedling). We excluded these from analyses that required knowledge of native/introduced status, but otherwise included them in indicator species analyses and when determining total plot species richness and abundance. We statistically tested effects of deer access/exclusion and deer density on richness and abundance of unknown species [see Supporting Information—Table S2]. We standardized species taxonomy and native/introduced status according to the United States Department of Agriculture Plants Database (USDA NRCS 2012). Taxa with status code of ‘Native and Introduced’ (i.e. some infra-taxa are native and some are introduced) were classified as native. We define introduced plants as invasive according to the USDA Forest Service (1998) and the USDA NRCS (2012).
Statistical analysis
We used mixed effects linear regression to test for effects of deer on relationships between native and introduced species richness and abundance. Introduced vegetation (i.e. species richness, cover or density) was the response variable, native vegetation and deer access/exclusion or deer population density were the fixed effects, and plot pair and site were the random effects. For the native vegetation effect in these models, native cover was used in the introduced cover analysis, native stem density in the introduced stem density analysis, and native species richness in the introduced species richness analysis. Deer density analyses were utilized only for unfenced, deer-access plot data here and below.
Deer effects on floristic composition, diversity and community-level abundance.
We used the multi-response permutation procedure (MRPP) (Mielke and Berry 2007) to test for community-level differences in floristic composition between deer-access and deer-exclusion plots using the Sørensen (Bray–Curtis) distance measure, which is not likely to exaggerate the influence of outliers in heterogeneous data, with PC-ORD software (McCune and Grace 2002). We conducted separate MRPPs for presence/absence and each abundance metric, cover and density. We calculated Shannon diversity (H′) (Shannon 1948), a combined measure of species richness and relative abundance (Hill 1973; McCune and Grace 2002), for each plot where at least one species was present. We calculated H′ for each abundance metric to determine floristic diversity using the equation:
where S is the total number of species measured according to each abundance metric and pi is the proportional abundance of species i in the plot.
We used linear mixed effects analysis of variance (ANOVA) to test for effects of deer access/exclusion and linear mixed effects regression to test for effects of deer population density on plant richness and absolute abundance of (i) native species, (ii) introduced species and (iii) total species (native plus introduced plus unknown species) and on Shannon diversity. Deer access/exclusion (fencing treatment) or deer population density were fixed effects and plot pair and site were random effects. We evaluated both absolute and proportion of introduced plant abundance (i.e. relative abundance) and plant species richness because they represent different indices of plant invasion; the former represents actual introduced plant abundance/species richness, while the latter represents the portion of plant community abundance/species richness composed of introduced plants. Absolute introduced plant abundance/species richness was evaluated based on the main effect of deer and proportion introduced plant abundance/species richness was evaluated based on the interaction of the deer effect with total vegetation. A significant interaction indicates that the ratio of introduced to total plant abundance/species richness (i.e. proportion introduced) varies with the deer effect. The ratio of introduced to native vegetation provides another index of plant invasion and was evaluated by testing the interaction of the deer effect with native vegetation. A significant interaction would indicate that the ratio of introduced to native vegetation varies with the deer effect. Total cover could exceed 100 % due to overlapping leaves of different species. We excluded sites lacking introduced plants from community-level mixed model analyses with introduced plants in the response variable.
We acknowledge that accurate deer density estimation is particularly difficult in forests (Putman et al. 2011). Total population counts can underestimate the actual number of deer by a factor of four or more (Andersen 1961). While distance sampling (Buckland et al. 1993, 2001), used to inform many of the estimates included in analyses here [see Supporting Information—Table S1], is a more accurate sampling approach vs. total population counts, considerable error surrounds single estimates and cannot fully account for season-to-season or year-to-year fluctuations or legacy effects of previous deer populations. Additionally, only deer-access plot data were used in these analyses; thus, the paired-plot baseline provided by fenced-plot data is lacking. Due to these limitations, we exercised caution in interpreting results of regression analyses. All sites were included in deer density analyses except West Point, for which deer population density estimates were unavailable.
Deer effects on individual introduced and native species’ abundance.
To follow up the MRPP and determine which species might be driving community-level differences, we used indicator species analysis (ISA) to test for species and genera affinities for deer access or deer exclusion (Dufrêne and Legendre 1997). The ISA results show which plant species or genera associate with deer-access or with deer-exclusion plots. We calculated indicator values for each species by multiplying the relative abundance across all plots by the relative frequency across plots within each treatment. We used a Monte Carlo randomization test to determine significance of indicator values, which range from 0 (not detected) to 100 (exclusive association). We conducted separate ISAs for presence/absence, cover and density data. We used PC-ORD software for the ISAs (McCune and Grace 2002). We report species as significantly associated with a treatment when α < 0.05.
We used linear mixed effects ANOVA to test the main effect of deer access/exclusion on abundance of individual introduced and native plant species. We conducted these species-level abundance analyses for the most frequently occurring introduced plants (defined here as species present in >5 % of all plots; a total of 13 introduced species) and for the 20 most frequent native plant species (present in >12 % of all plots). Plot pair and site were random effects included in models to control for within- and between-site variability, respectively.
Non-linearities are pervasive in ecology (e.g. Lockwood et al. 2013; Turner and Gardner 2015), yet we did not analyse them in the data presented here, opting instead to transform the data and test for linear patterns. For community- and species-level mixed models, we used square root or natural log transformations of response variables when necessary to meet statistical assumptions of normality and homogeneity of residuals. In community-level analyses, we report 95 % confidence intervals for significant fixed effects (α < 0.05) and, for mixed effect models with a significant deer treatment effect (α < 0.05), we determined least square means using t-tests (based on the Satterthwaite approximation for denominator degrees of freedom). To determine significance of random factors, we used log-likelihood ratio tests (chi-square with one degree of freedom, i.e. one effect tested at a time). We used the lme4 (Bates et al. 2015), lmerTest (Kuznetsova et al. 2013) and vegan (Oksanen et al. 2013) packages for mixed model analyses in R version 3.1.2 (R Development Core Team 2014). We report plot-level means and standard errors.
Results
We recorded 50 introduced and 345 native species in the regional forest understory species pool. Fifty-four species, six of which were introduced, only occurred in deer-access plots. In contrast, 72 species, 16 of which were introduced, only occurred in deer-exclusion plots. Of the introduced species, 32 % occurred only in deer-exclusion plots; 16 % of native species occurred only in deer-exclusion plots. A higher proportion of native species occurred in both deer-access and deer-exclusion plots (70 %) than of introduced species (56 %). Introduced and native species richness and abundance were significantly positively correlated (Table 2; see Supporting Information—Fig. S2). We detected no effect of deer on the ratio of introduced to native vegetation (non-significant interactions between deer effect and native species vegetation) (Table 2). At five sites, no introduced species were observed. Total species richness was 23 % higher at sites where introduced species were present vs. where they were absent. For species richness and abundance by deer access/exclusion treatment and site, see Supporting Information—Tables S4 and S5, respectively.
Table 2.
Mixed model effects of white-tailed deer a) access/exclusion and b) population density and native vegetation on introduced plant richness and abundance (per cent cover and stem density)a. Results are based on floristic composition data collected from deer-access (unfenced) and deer-exclusion (fenced) plots at 23 sites in east-central and north-eastern USA. The ratio of introduced to native vegetation was evaluated based on the interaction of the deer effect with native vegetation; the lack of significant interactions indicates that the ratio of introduced to native vegetation does not vary with the deer effect. See Supporting Information—Fig. S2 for the relationships between introduced and native vegetation. For random effect results, see Supporting Information—Table S3. P values are in bold print if significant at the alpha level α < 0.05.
| Introduced species richness | Introduced cover | Introduced stem density | |
|---|---|---|---|
| a) Deer access/exclusion | |||
| Intercept (SE) | 0.8 (0.2) | 1.3 (0.2) | 1.2 (0.3) |
| DA/DE coefficient (SE) | –0.01 (0.08) | –0.2 (0.1) | 0.05 (0.1) |
| F statistic (DFn,DFd) | 0.028 (1,222) | 2.2 (1,193) | 0.22 (1,171) |
| P value | 0.9 | 0.1 | 0.6 |
| Native vegetation coefficient (SE) | 0.036 (0.006) | 0.012 (0.005) | 0.020 (0.006) |
| F statistic (DFn,DFd) | 39 (1,388) | 11 (1,346) | 9.3 (1,257) |
| P value | <0.001 | <0.001 | 0.002 |
| DA/DE * Native vegetation coefficient (SE) | –5 × 10−4 (0.005) | –0.003 (0.004) | –0.010 (0.007) |
| F statistic (DFn,DFd) | 0.008 (1,216) | 0.56 (1,228) | 1.9 (1,194) |
| P value | 0.9 | 0.4 | 0.2 |
| n | 404 | 392 | 290 |
| # Sites | 18 | 17 | 11 |
| b) Deer density | |||
| Intercept (SE) | 1.3 (0.3) | 1.1 (0.6) | 0.5 (0.7) |
| DD coefficient (SE) | 0.003 (0.005) | –3 × 10−4 (0.01) | 0.01 (0.01) |
| F statistic (DFn,DFd) | 0.54 (1,23) | 0.0011 (1,16) | 1.4 (1,11) |
| P value | 0.5 | 1 | 0.3 |
| Native vegetation coefficient (SE) | 0.013 (0.008) | 0.023 (0.009) | 0.03 (0.02) |
| F statistic (DFn,DFd) | 2.3 (1,154) | 6 (1,127) | 2.8 (1,140) |
| P value | 0.1 | 0.01 | 0.09 |
| DD * Native vegetation coefficient (SE) | 3 × 10−4 (2 × 10−4) | 1 × 10−4 (3 × 10−4) | –3 × 10−4 (2 × 10−4) |
| F statistic (DFn,DFd) | 2.4 (1,185) | 0.32 (1,177) | 1.7 (1,139) |
| P value | 0.1 | 0.6 | 0.2 |
| n | 190 | 184 | 145 |
| # Sites | 17 | 16 | 11 |
aNative species richness was used as the native vegetation predictor variable for introduced species richness and native species cover and stem density were used as the native vegetation predictor variables for introduced cover and stem density, respectively. Square-root transformations of species richness and natural log +1 transformations of species cover and stem density were used to meet statistical assumptions. SE = standard error; DA = deer access; DE = deer exclusion; DFn = degrees of freedom, numerator; DFd = degrees of freedom, denominator; n = number of observations; DD = deer density.
Deer effects on floristic composition, diversity and community-level abundance
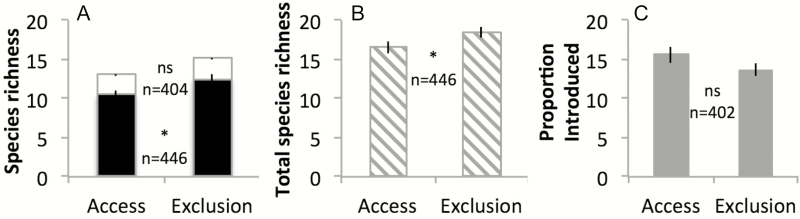
Species composition was significantly different between deer-access and deer-exclusion plots based on all three MRPP analyses despite high heterogeneity among plots within each treatment (Table 3). Deer-access plots had lower Shannon diversity (H′) than deer-exclusion plots (Table 4a; Fig. 2) and, among deer-access plots, H′ (cover but not density) was negatively correlated with deer density (Table 4b). Deer exclusion did not affect introduced plant species richness or the proportion of introduced plant species (non-significant interaction between deer access/exclusion and total species richness) (Table 5a; Fig. 3). However, as deer density increased, the proportion of introduced species increased (significant interaction between deer density and total species richness) (Table 5b). Deer-access plots had 16 % lower native plant species richness and 10 % lower total plant species richness than deer-exclusion plots (Table 5a; Fig. 3).
Table 3.
Results of MRPPs, testing the effect of white-tailed deer on species composition in east-central and north-eastern USA. Separate analyses were conducted for species presence/absence and abundance, per cent cover or stem density. The agreement statistic, A, indicates within-group homogeneity compared to random; A varies between 0 (heterogeneous plots) and 1 (homogenous plots). The P value and the number of plots within each group, deer access or deer exclusion, are shown. The number of plots was constrained in analyses due to plots with zero vegetation [see Supporting Information—Text S1].
| A | P value | Number of plots | ||
|---|---|---|---|---|
| Deer access | Deer exclusion | |||
| Presence/absence | 0.0019 | <0.001 | 221 | 223 |
| Abundance (cover) | 0.0027 | <0.001 | 185 | 188 |
| Abundance (density) | 0.0020 | 0.001 | 158 | 167 |
Table 4.
Mixed model effects of white-tailed deer a) access/exclusion and b) population density on introduced, native and total plant density, cover and Shannon diversity (H′) based on floristic composition data collected from deer-access and deer-exclusion plots in east-central and north-eastern USAa. Proportion introduced plant abundance was evaluated based on the interaction of the deer effect with total vegetation; a significant interaction indicates that the ratio of introduced to total plant abundance (i.e. proportion introduced) varies with the deer effect. The number of plots was constrained in the Shannon diversity analyses due to plots with zero vegetation [see Supporting Information—Text S1]. For random effect results, see Supporting Information—Table S3. P values and LSmeans treatment test results are in bold print if significant at the alpha level α < 0.05.
| Community index | Introduced cover | Native cover | Total cover | Shannon diversity (cover) | Introduced stem density | Native stem density | Total stem density | Shannon diversity (density) |
|---|---|---|---|---|---|---|---|---|
| a) Deer access/exclusion | ||||||||
| Intercept (SE) | 0.4 (0.2) | 1.9 (0.3) | 2.6 (0.3) | 1.0 (0.2) | 0.8 (0.2) | 1.8 (0.2) | 2.3 (0.3) | 1.6 (0.2) |
| DA/DE coefficient (SE) | –0.01 (0.1) | 0.62 (0.08) | 0.3 (0.1) | 0.17 (0.05) | 0.03 (0.1) | 0.30 (0.06) | 0.27 (0.06) | 0.19 (0.04) |
| F statistic (DFn,DFd) | 0.02 (1,201) | 58 (1,216) | 9.4 (1,216) | 12 (1,194) | 0.13 (1,161) | 23 (1,167) | 19 (1,167) | 17 (1,161) |
| P value | 0.9 | <0.001 | 0.002 | <0.001 | 0.7 | <0.001 | <0.001 | <0.001 |
| LSmeans treatment test | – | DE > DA | DE > DA | DE > DA | – | DE > DA | DE > DA | DE > DA |
| DA estimate (LCI–UCI) | – | 1.9 (1.1–2.6) | 2.6 (2.0–3.3) | 1.2 (0.8–1.5) | – | 1.8 (1.3–2.2) | 2.3 (1.7–2.9) | 1.6 (1.2–2.0) |
| DE estimate (LCI–UCI) | – | 2.5 (1.8–3.2) | 3.0 (2.4–3.6) | 1.3 (1.0–1.7) | – | 2.1 (1.6–2.5) | 2.6 (1.9–3.2) | 1.8 (1.4–2.2) |
| Total vegetation coefficient (SE) | 0.032 (0.002) | – | – | – | 0.025 (0.002) | – | – | – |
| F statistic (DFn,DFd) | 397 (1,343) | – | – | – | 95 (1,225) | – | – | – |
| P value | <0.001 | – | – | – | <0.001 | – | – | – |
| DA/DE * Total vegetation coefficient (SE) | –0.011 (0.002) | – | – | – | –0.003 (0.003) | – | – | – |
| F statistic (DFn,DFd) | 42 (1,232) | – | – | – | 1.1 (1,178) | – | – | – |
| P value | <0.001 | – | – | – | 0.3 | – | – | – |
| n | 392 | 434 | 434 | 373 | 290 | 336 | 336 | 325 |
| # Sites | 17 | 22 | 22 | 22 | 11 | 14 | 14 | 14 |
| b) Deer density | ||||||||
| Intercept (SE) | 0.2 (0.3) | 3.0 (0.4) | 3.4 (0.4) | 1.7 (0.2) | –0.3 (0.5) | 1.8 (0.5) | 2.2 (0.6) | 1.7 (0.4) |
| DD coefficient (SE) | 0.001 (0.005) | –0.030 (0.009) | –0.020 (0.009) | –0.015 (0.005) | 0.018 (0.008) | 4 × 10−5 (0.008) | 0.002 (0.01) | –0.002 (0.006) |
| F statistic (DFn,DFd) | 0.081 (1,17) | 11 (1,18) | 5.3 (1,17) | 9.8 (1,18) | 4.7 (1,12) | 2.9 × 10−5 (1,12) | 0.026 (1,12) | 0.13 (1,12) |
| P value | 0.8 | 0.004 | 0.03 | 0.006 | 0.051 | 1 | 0.9 | 0.7 |
| Total vegetation coefficient (SE) | 0.026 (0.003) | – | – | – | 0.06 (0.01) | – | – | – |
| F statistic (DFn,DFd) | 78 (1,105) | – | – | – | 41 (1,126) | – | – | – |
| P value | <0.001 | – | – | – | <0.001 | – | – | – |
| DD * Total vegetation coefficient (SE) | 2.6 × 10−4 (6 × 10−5) | – | – | – | –5 × 10−4 (1 × 10−4) | – | – | – |
| F statistic (DFn,DFd) | 21 (1,152) | – | – | – | 18 (1,130) | – | – | – |
| P value | <0.001 | – | – | – | <0.001 | – | – | – |
| n | 184 | 205 | 205 | 173 | 145 | 168 | 168 | 158 |
| # Sites | 16 | 21 | 21 | 21 | 11 | 14 | 14 | 14 |
aNatural log +1 transformations of cover and stem density data were used to meet statistical assumptions. SE = standard error; DA = deer access; DE = deer exclusion; DFn = degrees of freedom, numerator; DFd = degrees of freedom, denominator; LCI = lower confidence interval; UCI = upper confidence interval; DD = deer density; n = number of observations.
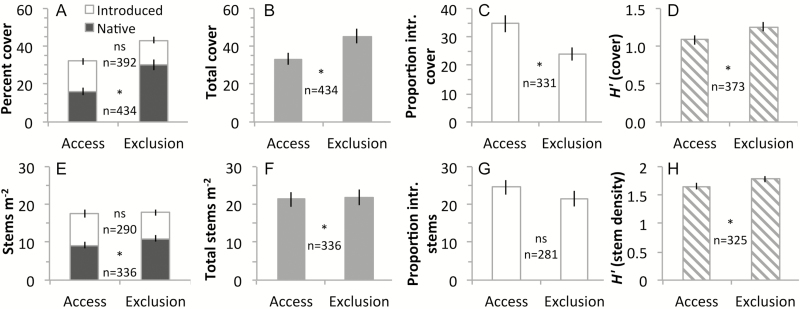
Figure 2.
Effects of white-tailed deer access/exclusion on (A, E) introduced and native plant abundance, (B, F) total plant abundance (includes unknown species), (C, G) proportion of introduced (intr.) plants and (D, H) Shannon Diversity (H′) in east-central and north-eastern USA. Means (±SE) are presented according to the abundance metric used for data collection, stem density (A–D) and/or cover (E–H) (see Table 1 for additional site information). An asterisk between bars indicates a significant effect of deer; ns = not significant; n = sample size (number of plots). The number of plots was constrained in the proportion introduced richness and Shannon diversity analyses due to plots with zero vegetation [see Supporting Information—Text S1].
Table 5.
Mixed model effects of white-tailed deer a) access/exclusion and b) population density on introduced, native and total plant species richness based on floristic composition data collected from deer-access (unfenced) and deer-exclusion (fenced) plots at 23 sites in east-central and north-eastern USAa. Proportion introduced plant species richness was evaluated based on the interaction of the deer effect with total species richness; a significant interaction indicates that the ratio of introduced to total plant species richness (i.e. proportion introduced) varies with the deer effect. For random effect results, see Supporting Information—Table S3. P values and LSmeans treatment test results are in bold print if significant at the alpha level α < 0.05.
| Introduced species richness | Native species richness | Total species richness | |
|---|---|---|---|
| a) Deer access/exclusion | |||
| Intercept (SE) | 0.4 (0.2) | 3.2 (0.2) | 4.0 (0.3) |
| DA/DE coefficient (SE) | –0.04 (0.07) | 0.39 (0.06) | 0.32 (0.06) |
| F statistic (DFn,DFd) | 0.31 (1,220) | 46 (1,222) | 25 (1,222) |
| P value | 0.6 | <0.001 | <0.001 |
| LSmeans treatment test | – | DE > DA | DE > DA |
| DA estimate (LCI–UCI) | – | 3.2 (2.7–3.8) | 4.0 (3.4–4.5) |
| DE estimate (LCI–UCI) | – | 3.6 (3.1–4.1) | 4.3 (3.7–4.8) |
| Total species richness coefficient (SE) | 0.044 (0.004) | – | – |
| F statistic (DFn,DFd) | 160 (1,397) | – | – |
| P value | <0.001 | – | – |
| DA/DE * Total species richness coefficient (SE) | 2 × 10−4 (0.003) | – | – |
| F statistic (DFn,DFd) | 0.004 (1,219) | – | – |
| P value | 0.9 | – | – |
| n | 404 | 446 | 446 |
| # Sites | 18 | 23 | 23 |
| b) Deer density | |||
| Intercept (SE) | 0.2 (0.4) | 3.9 (0.4) | 4.5 (0.5) |
| DD coefficient (SE) | 0.005 (0.007) | –0.020 (0.009) | –0.01 (0.01) |
| F statistic (DFn,DFd) | 0.64 (1,22) | 5.1 (1,19) | 2.1 (1,19) |
| P value | 0.4 | 0.04 | 0.2 |
| Total species richness coefficient (SE) | 0.031 (0.008) | – | – |
| F statistic (DFn,DFd) | 15 (1,183) | – | – |
| P value | <0.001 | – | – |
| DD * Total species richness coefficient (SE) | 3 × 10−4 (1 × 10−4) | – | – |
| F statistic (DFn,DFd) | 3.9 (1,185) | – | – |
| P value | 0.049 | – | – |
| n | 190 | 211 | 211 |
| # Sites | 17 | 22 | 22 |
aSquare-root transformations of species richness were used to meet the assumption of homogeneity of residuals. SE = standard error; DA = deer access; DE = deer exclusion; DFn = degrees of freedom, numerator; DFd = degrees of freedom, denominator; LCI = lower confidence interval; UCI = upper confidence interval; n = number of observations; DD = deer density.
Figure 3.
Effects of white-tailed deer access/exclusion on mean (±SE) (A) introduced (white shading) and native (black shading) plant species richness, (B) total plant species richness (includes unknown species) and (C) proportion introduced plant species richness in east-central and north-eastern USA. An asterisk between bars indicates a significant effect of deer; ns = not significant; n = sample size (number of plots). The number of plots was constrained in the proportion introduced richness analysis due to plots with zero vegetation [see Supporting Information—Text S1].
While deer-access plots tended to have higher absolute introduced plant abundance than deer-exclusion plots, these trends were not statistically significant (Fig. 2; Table 4). However, in deer-access plots, native stem density was 16 % lower and native cover was 46 % lower than in deer-exclusion plots (Fig. 2; Table 4). Total stem density was 2 % lower and total cover was 27 % lower in deer-access plots than in deer-exclusion plots. The proportion of introduced plant cover was 44 % higher in deer-access vs. deer-exclusion plots (Fig. 2; Table 4a). The proportion of introduced plant stems was numerically, but not statistically, higher in deer-access plots (Fig. 2; Table 4a). The proportion of introduced plant abundance (cover and stem density) was positively correlated with deer density, while native and total plant cover were negatively correlated with deer density (Table 4b). In summary, deer had no effect on absolute introduced abundance but they increased the proportion composed of introduced species. The difference between the absolute and proportional metrics is native vegetation, which is reduced by deer. Thus, deer indirectly increase the proportion of introduced vegetation via their negative influence on native vegetation. Notably, we detected these effects after accounting for within- and between-site variability, which were significant random factors [see Supporting Information—Table S3]. More unknown species occurred in deer-access plots than in deer-exclusion plots but unknown species abundance was unaffected [see Supporting Information—Table S2].
Deer effects on individual introduced and native species’ abundance
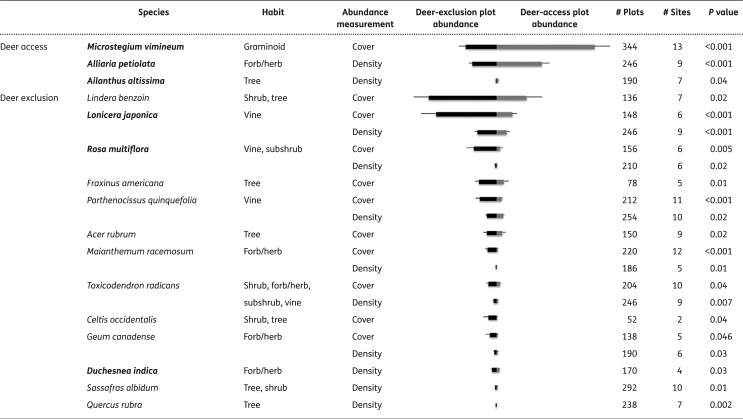
Deer access/exclusion differentially affected introduced and native plant species. Indicator species analysis results showed that three introduced species and four native species were indicators of deer-access plots, while three introduced and 15 native species were indicators of deer-exclusion plots (Table 6). Two introduced plants, the grass Microstegium vimineum and the herb Alliaria petiolata, occurred in a large number of plots and sites and were the best indicator species (by indicator value) of deer-access plots (Table 6). Indicator species of deer-exclusion plots included the introduced vine Lonicera japonica and shrub Rosa multiflora (Table 6). In general, introduced indicator species were found to be more abundant in their respective deer-access or deer-exclusion plots using mixed model analyses (Table 7). In deer-access plots, absolute abundance of three introduced species, M. vimineum, A. petiolata and the tree Ailanthus altissima, was higher than in deer-exclusion plots. In contrast, three other introduced species, L. japonica, R. multiflora and Duchesnea indica, occurred in lower abundance in deer-access vs. deer-exclusion plots (Table 7).
Table 6.
Indicator species analysis results showing plant species and genera associated with deer access or with deer exclusion in east-central and north-eastern USA. Introduced species are in bold type. Indicator values range from 0 (no indication of association with treatment) to 100 (perfect indication) and were determined according to species’ presence/absence (p/a) and the metric used to record abundance, density and/or cover. The number of plots and sites where each species was observed is included to indicate frequency and distribution across the 23 sites analysed. Results are arranged by deer access/exclusion, then by indicator value and then by P value; each species’ results are listed together.
| Species | Habita | Abundance measurement | Indicator value | P value | # Plots | # Sites | |
|---|---|---|---|---|---|---|---|
| Deer access | Microstegium vimineum | Graminoid | Cover | 35 | <0.001 | 146 | 13 |
| p/a | 23 | 0.02 | 148 | 14 | |||
| Alliaria petiolata | Forb/herb | Density | 29 | 0.02 | 133 | 9 | |
| Polygonum | Density | 13 | 0.05 | 47 | 7 | ||
| Pilea pumila | Forb/herb | Density | 11 | 0.01 | 33 | 5 | |
| p/a | 8 | 0.04 | 40 | 9 | |||
| Oxalis | Density | 8 | 0.04 | 26 | 6 | ||
| Oxalis stricta | Forb/herb | Density | 8 | 0.02 | 21 | 4 | |
| Perilla frutescens | Forb/herb | p/a | 6 | 0.04 | 26 | 7 | |
| Acalypha rhomboidea | Forb/herb | Density | 5 | 0.009 | 9 | 2 | |
| p/a | 3 | 0.02 | 9 | 2 | |||
| Cinna arundinacea | Graminoid | Cover | 3 | 0.03 | 7 | 2 | |
| Prenanthes | Cover | 3 | 0.04 | 7 | 3 | ||
| Solanum | Density | 3 | 0.05 | 4 | 4 | ||
| Deer exclusion | Lonicera japonica | Vine | Density | 25 | 0.05 | 118 | 9 |
| Cover | 16 | 0.01 | 67 | 6 | |||
| Parthenocissus quinquefolia | Vine | Cover | 25 | 0.01 | 111 | 11 | |
| Prunus serotina | Tree, shrub | p/a | 24 | 0.009 | 159 | 19 | |
| Toxicodendron radicans | Shrub, forb/herb, subshrub, vine | Density | 20 | 0.04 | 86 | 9 | |
| Rosa multiflora | Vine, subshrub | p/a | 14 | 0.03 | 81 | 11 | |
| Cover | 8 | 0.03 | 28 | 6 | |||
| Maianthemum racemosum | Forb/herb | Cover | 13 | <0.001 | 42 | 12 | |
| p/a | 12 | 0.007 | 61 | 16 | |||
| Density | 8 | 0.02 | 23 | 5 | |||
| Ulmus rubra | Tree | Density | 13 | 0.008 | 45 | 8 | |
| p/a | 12 | 0.005 | 56 | 9 | |||
| Rubus | p/a | 13 | 0.03 | 77 | 13 | ||
| Viburnum acerifolium | Shrub, subshrub | Cover | 11 | 0.001 | 28 | 8 | |
| p/a | 10 | 0.001 | 37 | 10 | |||
| Carya cordiformis | Tree | Density | 11 | 0.004 | 31 | 8 | |
| p/a | 10 | 0.01 | 47 | 13 | |||
| Quercus rubra | Tree | Density | 11 | 0.01 | 33 | 7 | |
| Polygonatum biflorum | Forb/herb | Cover | 10 | 0.03 | 30 | 10 | |
| p/a | 8 | 0.02 | 37 | 13 | |||
| Carya alba | Tree | p/a | 6 | 0.03 | 28 | 10 | |
| Density | 6 | 0.03 | 17 | 7 | |||
| Cover | 5 | 0.05 | 16 | 4 | |||
| Actaea racemosa | Forb/herb | Cover | 5 | 0.03 | 12 | 5 | |
| Rhododendron periclymenoides | Shrub | p/a | 4 | 0.02 | 10 | 4 | |
| Cover | 3 | 0.03 | 6 | 2 | |||
| Euthamia graminifolia | Forb/herb | Cover | 3 | 0.03 | 6 | 1 | |
| p/a | 3 | 0.03 | 6 | 1 | |||
| Circaea alpina | Forb/herb | Cover | 3 | 0.03 | 6 | 1 | |
| p/a | 3 | 0.03 | 6 | 1 | |||
| Lonicera maackii | Shrub | p/a | 3 | 0.01 | 7 | 3 | |
| Rubus pensilvanicus | Subshrub | p/a | 3 | 0.03 | 6 | 3 |
aThe native status based on genus alone is unknown.
Table 7.
Effects of deer on the abundance of the most frequent introduced (in bold type) and native plant species in east-central and north-eastern USA based on mixed models using floristic composition data collected from deer-access and deer-exclusion plots. Only significant effects are shown of the 13 introduced and 20 native species analysed. Abundance (+SE) in deer-access and deer-exclusion plots is presented; units for density are plants m−2 and for cover are per cent cover. Results are arranged by deer access/exclusion and then by abundance; each species’ results are listed together.
The native herbs Pilea pumila and Oxalis stricta were indicators of deer access, but others, including Maianthemum racemosum and Polygonatum biflorum were indicators of deer exclusion. Native trees P. serotina, Ulmus rubra, two Carya spp. and Quercus rubra and native shrubs Viburnum acerifolium and Rhododendron periclymenoides were indicator species of deer exclusion (Table 6). The cover of native trees Fraxinus americana, Acer rubrum and Celtis occidentalis and the native shrub Lindera benzoin was reduced in deer-access plots relative to deer-exclusion plots (Table 7). The native vines Parthenocissus quinquefolia and Toxicodendron radicans were indicators of deer exclusion and occurred in greater abundance in deer-exclusion vs. deer-access plots (Tables 6 and 7). Unknown species in the genera Polygonum, Oxalis, native Prenanthes and Solanum were associated with deer access, while unknown Rubus spp. indicated deer exclusion. For frequencies of each taxon recorded in deer-access plots, in deer-exclusion plots and overall, see Supporting Information—Table S6.
Discussion
White-tailed deer alter plant species composition and reduce community-wide plant diversity, upholding our first prediction. Deer facilitate some but not all introduced plant species and strongly negatively affect native plant species, offering partial support to our second prediction that deer would benefit introduced plants and disadvantage native plants. This work substantially clarifies previous conflicting reports of deer effects on introduced plants. By exploring deer-plant patterns across the region, our results provide evidence that attribute the seemingly contradictory findings in individual site-level studies to species-level differences, illustrating the presumed consequences of differential palatability to deer (Senft et al. 1987; Keane and Crawley 2002; Averill et al. 2016). Our results are consistent with previous research showing that deer can increase (Knight et al. 2009; Eschtruth and Battles 2009b; Beasley and McCarthy 2011; Kalisz et al. 2014; Dávalos et al. 2015b), decrease (Rossell et al. 2007; Shelton et al. 2014), have no effect (Bowers 1993; Levine et al. 2012; DiTommaso et al. 2014) or mixed effects (Cadenasso et al. 2002; Webster et al. 2005; Knapp et al. 2008; Shen et al. 2016) on introduced plants. Where deer facilitate an increase in introduced plant abundance, plant invasion via enemy release (Elton 1958; Keane and Crawley 2002; Colautti et al. 2004) might be responsible. In contrast, where deer decrease introduced plants, biotic resistance to plant invasion is a possible outcome (Levine et al. 2004; Parker and Hay 2005; Parker et al. 2006). Despite within- and between-site heterogeneity, the fact that deer had negative impacts on native plants and indirect, facilitative effects on the proportion of introduced plant abundance elucidates the overarching effects of deer on vegetation at a regional scale (Russell et al. 2001).
That introduced and native species richness and abundance patterns are positively correlated (Table 2; see Supporting Information—Fig. S2) is consistent with research showing that introduced plant species invade ‘hot spots’ of diversity at large spatial scales (Stohlgren et al. 1999; Stark et al. 2006). Site characteristics, such as spatial heterogeneity in abiotic conditions (Davies et al. 2005), including land-use history and soil nutrients (Fraterrigo et al. 2006) across sites likely are responsible for the positive relationship between native and introduced plant richness and abundance.
Deer effects on floristic composition, diversity and community-level abundance
Deer do not directly impact introduced plant species richness (Table 5; Fig. 3) or abundance (Table 4; Fig. 2), which is evidence against our second prediction. These results are surprising, as many sites and large areas within the region and across the world currently are dominated by introduced species and also have high deer or other large herbivore populations (Waller and Alverson 1997; Rooney et al. 2004; Vavra et al. 2007). Such observations prompted our second prediction that greater richness and abundance of introduced plants would accompany deer access vs. deer exclusion. The fact that deer increase the proportion of cover of introduced plants appears to arise from the substantial decrease in the native flora imposed by deer. These results imply that the positive deer effect on the relative cover of introduced plants is caused indirectly by greater susceptibility of native vs. introduced plants to deer (however, see species-level results below). Deer have a markedly stronger negative effect on native species than on introduced species both in forest understories, as found in this work, and in an old field (DiTommaso et al. 2014). This result stands in contrast to reports that native and introduced species behave similarly in dynamic systems (Meiners 2007; Stromberg et al. 2009), albeit because of a native herbivore. The perspective that species be judged based on function and not on where they originated is gaining ground (Davis et al. 2011), yet our results show an important difference between native and introduced plants, namely their general susceptibility or response to herbivory, suggesting that native status has a deserved role in future research and in management decision-making. Our analyses suggest that declines in plant community diversity (McKinney and Lockwood 1999; Rooney et al. 2004) result more from deer herbivory than from the presence of introduced plants, a result also detected in other work (Morrison, this issue). Deer are a key driver of community change (Waller and Alverson 1997), while invasive plants are likely passengers opportunistically taking advantage of ecosystem alterations (MacDougall and Turkington 2005; Didham et al. 2007).
Our finding that deer increase the proportion of cover of introduced plants (Fig. 2) opposes the broadly observed biotic resistance pattern in which native herbivores reduce the relative abundance of introduced vegetation (Parker et al. 2006) as a result of differential palatability among introduced and native species (Parker and Hay 2005). This global meta-analysis found that native herbivores (e.g. insects, rodents and cervids) suppress introduced plants more than native plants. While informative for plant–herbivore interactions generally, such an extensive analysis is less likely to be predictive for a particular herbivore. Nonetheless, deer herbivory is a constant and important filter of regional species pools (Rooney et al. 2004) and could have a role in biotic resistance for certain introduced species (Maron and Vilà 2001), even preventing them from appearing in floristic census records.
Deer effects on individual introduced and native species’ abundance
Overall, we found a few graminoid and herbaceous species are favoured in the presence of deer, while trees, shrubs, vines and many herbaceous species lose out (Tables 6 and 7). These findings are consistent with assessments of winning and losing species in Northern Wisconsin (Rooney et al. 2004; Wiegmann and Waller 2006) and globally (McKinney and Lockwood 1999). Our finding that many woody and herbaceous plant species are negatively impacted by deer contrasts with results from a meta-analysis showing that woody, but not herbaceous species are negatively impacted by deer (Habeck and Schultz 2015), a discrepancy possibly owing to publication bias detected in the meta-analysis. Our work clearly shows that deer facilitate several notorious invasive plants in east-central and north-eastern USA, including A. altissima (tree-of-heaven), A. petiolata (garlic mustard) and M. vimineum (Japanese stilt-grass) (Tables 6 and 7). Positive effects of deer on A. petiolata and M. vimineum have been found in site-level experiments (Eschtruth and Battles 2009a, b; Knight et al. 2009; Kalisz et al. 2014; Dávalos et al. 2015a, b) and deer have been implicated in the establishment of A. altissima (Knapp and Canham 2000). The facilitative effect of deer on these species is likely due to their unpalatability relative to other plants. In deer preference trials, A. petiolata and M. vimineum were the least palatable of 15 introduced and native species (Averill et al. 2016). Ailanthus altissima is apparently also unpalatable (Forgione 1993), yet anecdotal evidence of browsing has been observed (K. L. Caraher, Hood College, pers. obs.) and thus the species’ rapid growth rate (Knapp and Canham 2000) could outweigh herbivory. These results show how unpalatable plants can gain an apparent competitive advantage relative to palatable plants (Holt 1977), i.e. native plants, and become more strongly represented in the flora or even invasive (Senft et al. 1987; Keane and Crawley 2002; Royo and Carson 2006; Arcese et al. 2014).
While deer facilitated an increase in the abundance of several unpalatable invaders in unfenced plots, deer exclusion in fenced plots resulted in higher abundance of several other invaders, including L. japonica (Japanese honeysuckle) and R. multiflora (multiflora rose) (Table 7). Lonicera japonica, L. maackii, and R. multiflora were indicator species of deer-exclusion plots (Table 6), reinforcing previous findings (Shelton et al. 2014) and suggesting these fleshy-fruited species perform better where protected against deer browsing. Even if species perform well enough where deer occur to be considered invasive, they might perform better where deer are excluded. These findings might be an outcome of one or several processes, three of which are outlined here. (i) These species are palatable (Sheldon and Causey 1974; Ashton and Lerdau 2008; Averill et al. 2016) and, in heavily browsed plant communities, the most palatable species are the most susceptible to being consumed and reduced in abundance (Royo and Carson 2006). Indeed, a decrease of L. japonica has been observed anecdotally in south-eastern Indiana as deer populations increased from the 1970s through 1990s (D. K. Apsley, The Ohio State University, pers. obs.). Tangentially, palatable invasive shrubs, such as L. maackii, which offers a leafy source of protein in early spring when native species are still leafless, might serve to boost deer populations (Martinod and Gorchov, this issue). (ii) Increased propagule pressure via bird-dispersal could account for the higher abundance of fleshy-fruited species observed in fenced plots. Birds are attracted to the additional habitat (e.g. food, shelter and perch points) and fences occurring where deer are excluded (McShea and Rappole 2000; Chollet and Martin 2013) and they are liable to disperse plant seeds via their droppings. Mutualistic interactions are of known importance in plant invasion (Richardson et al. 2000; (Gleditsch and Carlo 2011). (iii) Vines, such as L. japonica and the native P. quinquefolia, could be more abundant in fenced plots because they can climb on the more abundant vegetation occurring in deer-exclusion plots and on the fences themselves. The possibility of climbing was controlled experimentally at the two Valley Forge sites through the use of a metal stake placed in the centre of control plots (Abrams and Johnson 2012), yet the few occurrences of L. japonica and R. multiflora at the Valley Forge–Mt Joy (VJ) site were in deer-exclusion plots [see Supporting Information—Table S6], implicating deer exclusion as causal in increasing these vines’ abundance.
Deer have strong negative impacts on native species of many life forms. Overstory species, such as A. rubrum, Carya spp., F. americana and Quercus spp., appear to benefit from deer exclusion (Tables 6 and 7). Many other researchers (e.g. Abrams and Johnson 2012; Bressette et al. 2012; Nuttle et al. 2013; Abrams 2013; Owings et al., this issue) also report negative impacts of abundant deer on native tree species, implying that forest regeneration could be at risk. Shrubs, including L. benzoin, R. periclymenoides and V. acerifolium, also appear negatively influenced by deer (Tables 6 and 7), which jeopardizes organisms in other trophic levels that depend on forest understory shrub layers, e.g. birds (deCalesta 1994; McShea and Rappole 2000; Fuller 2001; Chollet and Martin 2013).
Site influences
Five sites were uninvaded by introduced plants. However, the native fern Dennstaedtia punctilobula, which is considered a native invasive plant (de la Cretaz and Kelty 1999), is dominant at two of the sites in north-eastern Pennsylvania (Long Run and Marienville). At the other three sites (Fernow, Monongahela and Zaleski), deer density estimates were considerably lower (~6 deer km−2) than the average across sites (mean = 35 deer km−2; median = 26 deer km−2) (Table 1). At Fernow and Monongahela, deer were shown to increase herbaceous richness and abundance by reducing fast-growing early successional species (Royo et al. 2010a). Thus, sites without introduced invaders might instead have native invaders or low deer densities, which might be associated with increased biotic resistance to introduced plant invasion.
In addition to deer density, overstory species composition and duration of deer exclusion varied among sites (Table 1) and likely contributed to varying deer effect patterns at the site level [see Supporting Information—Tables S4 and S5]. While sites were not selected randomly, most were not established to study invasive plants and spanned a wide deer abundance gradient (Table 1). Furthermore, many concomitant and often interactive factors not limited to deer and invasive plants (e.g. forest successional age and proximity to centres of human activity, propagule pressure, resource availability, invasive earthworms, etc.) affect forest understory diversity (Abrams 1998; Pysřek et al. 2002) Baiser et al. 2008; Eschtruth and Battles 2009b; Royo et al. 2010a; Martin and Baltzinger 2011; Fisichelli et al. 2013; Dávalos et al. 2014; Dobson and Blossey 2015; Forsyth et al. 2015; Nuzzo et al. 2015), yet were not included in analyses here. Including such factors in future work would improve understanding of community assembly and invasion processes. The influence of some site characteristics, including surrounding landscape structure and composition, on the relationship between deer and plant invasion is explored elsewhere (Averill 2014).
In floristic censuses, plant abundance is sometimes sampled using different metrics for different plant habits (Table 1), which presents issues for pooled or meta-analysis, such as requiring analysis and interpretation of multiple abundance metrics. The results reported here also show that using different abundance metrics can yield different results. For example, deer access increased the proportion of introduced plant cover, but not stem density (Table 4; Fig. 2), perhaps because herbivory influences cover more than stem density. Furthermore, determining total stem density or total cover, and therefore total vegetation abundance, depends on species being sampled in the same way. Total plant abundance is a useful metric for relating primary productivity to ecosystem functioning (Chapin et al. 2002), but cannot be calculated in data sets that use different abundance metrics for different plant habits, as was the case here.
Conclusions
This analysis deepens ecological understanding of some key factors in the invasion process. In this work, an abundant, native, large herbivore is shown to alter plant community composition, lower diversity, reduce native plant richness and abundance, and increase the relative cover of introduced plants. Unpalatable invasive plants seem to benefit under heavy herbivore pressure. While introduced plant invasion has been causally implicated in native plant decline (Wiegmann and Waller 2006), ruminant herbivory appears to be a key factor affecting both processes. Dominant native herbivores such as deer are important agents of ecosystem change as their presence (i) reduces native biodiversity and (ii) increases the relative abundance of introduced plants, two of the major drivers affecting modern plant communities and ecosystems (Hooper et al. 2012).
Supporting Information
The following additional information is available in the online version of this article—
Text S1. Additional vegetation data processing methods.
Table S1. Sources and methods for deer density estimates.
Table S2. Statistical results for effects of deer on unknown species.
Table S3. Statistical results showing the influence of the random effects plot and site.
Table S4. Species richness by plant native status, deer access/deer exclusion and site.
Table S5. Vegetation abundance by metric used (per cent cover or stem density), plant native status, deer access/deer exclusion and site.
Table S6. Taxa frequency by deer access/deer exclusion and plant native status, with sites of occurrence.
Figure S1. Vegetation cover class categories used to estimate plant abundance across 15 sites.
Figure S2. Relationships between introduced and native species richness and abundance.
Sources of Funding
This work was funded by: the United States Department of Agriculture National Needs Program (K.M.A. and D.A.M.), Penn State College of Agricultural Sciences (K.M.A.), National Science Foundation (NSF) awards DEB 1457531 and DEB 0958676 (S.K.), the NSF award DBI 0851303 and DBI 1156799 (J.D.P.), the Cooperative Agreement H399206006 from the National Park Service (J.D.P.), the U.S. Forest Service award RWU NE-4557 (through agreement JV-11242328-121 with Hood College) (D.H.B. and K.L.C.), the U.S. Department of Energy (DOE) Fermilab National Environmental Research Park (operated by Fermi Research Alliance, LLC under Contract No. DE-AC02-07CH11359 with the US DOE) (V.A.N.) and the Strategic Environmental Research and Development Program (SERDP) of the U.S. Department of Defense (Grant RC-1542) (B.B.).
Contributions by the Authors
K.M.A., D.A.M., E.A.H.S., S.K., W.J.M., N.A.B. and J.D.P. conceived of the project. A.A.R. established Long Run and Marienville deer exclusion experimental sites, which were re-sampled by K.M.A. and D.A.M. K.M.A., D.A.M., S.K., W.J.M., N.A.B., J.D.P., M.D.A., D.K.A., B.B., D.H.B., K.L.C., S.E.J., R.M. and V.A.N. established experiments and/or collected data. S.E.J. created Fig. 1. K.M.A. analysed the data and wrote the manuscript. All authors contributed to revisions.
Conflicts of Interest
None declared.
Supplementary Material
Acknowledgements
We are grateful to the parks that cooperated with us for data collection, the many who helped collect plant community data, including K. Barlow, T. Isabel, J. Snitzer, K. Kyde, B. Sands, C. Caceras, D. Yeh, D. Weller, E. Lind, G. Sivak, J. Miguel, J. Shue, K. Edson and M. Muñiz. The authors are thankful to T. Hutchinson (U.S. Forest Service) for sharing plant community data. We thank F. Vermeylen (Cornell University) for statistical advice. We appreciate feedback on previous versions of this article from K. Shea, E. Post, M. Ryan, AoB PLANTS guest editor D. Gorchov and anonymous reviewers.
Literature Cited
- Abrams MD. 1998. The red maple paradox. BioScience 48:355–364. [Google Scholar]
- Abrams MD. 2013. The impact of mast years on seedling recruitment following canopy thinning and deer fencing in contrasting northeastern U.S. coastal forests. The Journal of the Torrey Botanical Society 140:379–390. [Google Scholar]
- Abrams MD, Johnson SE. 2012. Long-term impacts of deer exclosures on mixed-oak forest composition at the Valley Forge National Historical Park, Pennsylvania, USA. The Journal of the Torrey Botanical Society 139:167–180. [Google Scholar]
- Alroy J. 2008. Dynamics of origination and extinction in the marine fossil record. Proceedings of the National Academy of Sciences 105:11536–11542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alverson WS, Waller DM, Solheim SL. 1988. Forests too deer: edge effects in northern Wisconsin. Conservation Biology 2:348–358. [Google Scholar]
- Andersen J. 1961. Biology and management of roe-deer in Denmark. La Terre et la Vie 108:41–53. [Google Scholar]
- Arcese P, Schuster R, Campbell L, Barber A, Martin TG. 2014. Deer density and plant palatability predict shrub cover, richness, diversity and aboriginal food value in a North American archipelago. Diversity and Distributions 20:1368–1378. [Google Scholar]
- Ashton IW, Lerdau MT. 2008. Tolerance to herbivory, and not resistance, may explain differential success of invasive, naturalized, and native North American temperate vines. Diversity and Distributions 14:169–178. [Google Scholar]
- Asner GP, Elmore AJ, Olander LP, Martin RE, Harris AT. 2004. Grazing systems, ecosystem responses, and global change. Annual Review of Environment and Resources 29:261–299. [Google Scholar]
- Augustine DJ, McNaughton SJ. 1998. Ungulate effects on the functional species composition of plant communities: herbivore selectivity and plant tolerance. The Journal of Wildlife Management 62:1165–1183. [Google Scholar]
- Averill KM. 2014. The influence of white-tailed deer and landscape composition and structure on exotic plant success. PhD Dissertation, The Pennsylvania State University, University Park, PA. [Google Scholar]
- Averill KM, Mortensen DA, Smithwick EAH, Post E. 2016. Deer feeding selectivity for invasive plants. Biological Invasions 18:1247–1263. [Google Scholar]
- Baiser B, Lockwood JL, Puma D, Aronson MFJ. 2008. A perfect storm: two ecosystem engineers interact to degrade deciduous forests of New Jersey. Biological Invasions 10:785–795. [Google Scholar]
- Bartuszevige AM, Endress BA. 2008. Do ungulates facilitate native and exotic plant spread?: seed dispersal by cattle, elk and deer in northeastern Oregon. Journal of Arid Environments 72:904–913. [Google Scholar]
- Bates S. 2009. National Capital Region Network 2007 deer monitoring report. National Park Service Published Report-661246, Fort Collins, CO. [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67:1–48. [Google Scholar]
- Beasley RR, McCarthy BC. 2011. Effects of Microstegium vimineum (Trin.) A. Camus (Japanese stiltgrass) on native hardwood survival and growth: implications for restoration. Natural Areas Journal 31:246–255. [Google Scholar]
- Blettner M, Sauerbrei W, Schlehofer B, Scheuchenpflug T, Friedenreich C. 1999. Traditional reviews, meta-analyses and pooled analyses in epidemiology. International Journal of Epidemiology 28:1–9. [DOI] [PubMed] [Google Scholar]
- Bowers MA. 1993. Influence of herbivorous mammals on an old-field plant community: years 1–4 after disturbance. Oikos 67:129–141. [Google Scholar]
- Bressette JW, Beck H, Beauchamp VB. 2012. Beyond the browse line: complex cascade effects mediated by white-tailed deer. Oikos 121:1749–1760. [Google Scholar]
- Brown ML, Brown RG. 1984. Herbaceous plants of Maryland. Baltimore, MD: Port City Press. [Google Scholar]
- Buckland ST, Anderson DR, Burnham KP, Laake JL. 1993. Distance sampling: estimating abundance of biological populations. London, UK: Chapman and Hall. [Google Scholar]
- Buckland ST, Anderson DR, Burnham KP, Laake JL, Borchers DL, Thomas L. 2001. Introduction to distance sampling: estimating abundance of biological populations. Oxford, UK: Oxford University Press. [Google Scholar]
- Cadenasso ML, Pickett STA, Morin PJ. 2002. Experimental test of the role of mammalian herbivores on old field succession: community structure and seedling survival. Journal of the Torrey Botanical Society 129:228–237. [Google Scholar]
- Caraher KL. 2009. White-tailed deer herbivory facilitates increased abundance of introduced plants beneath forest canopy gaps. MS Thesis, Hood College, MD. [Google Scholar]
- Castellano SM, Gorchov DL. 2013. White-tailed deer (Odocoileus virginianus) disperse seeds of the invasive shrub, amur honeysuckle (Lonicera maackii). Natural Areas Journal 33:78–80. [Google Scholar]
- Chapin FSI, Matson PA, Mooney HA. 2002. Principles of terrestrial ecosystem ecology. New York: Springer. [Google Scholar]
- Chollet S, Martin J-L. 2013. Declining woodland birds in North America: should we blame Bambi?Diversity and Distributions 19:481–483. [Google Scholar]
- Clark JS, Beckage B, Camill P, Cleveland B, HilleRisLambers J, Lichter J, McLachlan J, Mohan J, Wyckoff P. 1999. Interpreting recruitment limitation in forests. American Journal of Botany 86:1 –16. [PubMed] [Google Scholar]
- Clark JS, Carpenter SR, Barber M, Collins S, Dobson A, Foley JA, Lodge DM, Pascual M, Pielke R Jr, Pizer W, Pringle C, Reid WV, Rose KA, Sala O, Schlesinger WH, Wall DH, Wear D. 2001. Ecological forecasts: an emerging imperative. Science 293:657 –660. [DOI] [PubMed] [Google Scholar]
- Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ. 2004. Is invasion success explained by the enemy release hypothesis?Ecology Letters 7:721–733. [Google Scholar]
- Cook-Patton SC, LaForgia M, Parker JD. 2014. Positive interactions between herbivores and plant diversity shape forest regeneration. Proceedings of the Royal Society of London B: Biological Sciences 281:20140261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté SD, Rooney TP, Tremblay J-P, Dussault C, Waller DM. 2004. Ecological impacts of deer overabundance. Annual Review of Ecology, Evolution, and Systematics 35:113–147. [Google Scholar]
- Crawley M. 1997. Plant ecology, 2nd edn.Cambridge, UK: University Press. [Google Scholar]
- de la Cretaz AL, Kelty MJ. 1999. Establishment and control of hay-scented fern: a native invasive species. Biological Invasions 1:223–236. [Google Scholar]
- Dávalos A, Nuzzo V, Blossey B. 2014. Demographic responses of rare forest plants to multiple stressors: the role of deer, invasive species and nutrients. Journal of Ecology 102:1222–1233. [Google Scholar]
- Dávalos A, Nuzzo V, Blossey B. 2015a. Interactive effects of deer, earthworms and non-native plants on rare forest plant recruitment. Biological Conservation 187:173–181. [Google Scholar]
- Dávalos A, Nuzzo V, Blossey B. 2015b. Single and interactive effects of deer and earthworms on non-native plants. Forest Ecology and Management 351:28–35. [Google Scholar]
- Davies KF, Chesson P, Harrison S, Inouye BD, Melbourne BA, Rice KJ. 2005. Spatial heterogeneity explains the scale dependence of the native–exotic diversity relationship. Ecology 86:1602–1610. [Google Scholar]
- Davis MA, Chew MK, Hobbs RJ, Lugo AE, Ewel JJ, Vermeij GJ, Brown JH, Rosenzweig ML, Gardener MR, Carroll SP, Thompson K, Pickett STA, Stromberg JC, Tredici PD, Suding KN, Ehrenfeld JG, Philip Grime J, Mascaro J, Briggs JC. 2011. Don’t judge species on their origins. Nature 474:153–154. [DOI] [PubMed] [Google Scholar]
- deCalesta DS. 1994. Effect of white-tailed deer on songbirds within managed forests in Pennsylvania. The Journal of Wildlife Management 58:711–718. [Google Scholar]
- Didham RK, Tylianakis JM, Gemmell NJ, Rand TA, Ewers RM. 2007. Interactive effects of habitat modification and species invasion on native species decline. Trends in Ecology & Evolution 22:489–496. [DOI] [PubMed] [Google Scholar]
- DiTomaso JM. 2000. Invasive weeds in rangelands: species, impacts, and management. Weed Science 48:255–265. [Google Scholar]
- DiTommaso A, Morris SH, Parker JD, Cone CL, Agrawal AA. 2014. Deer browsing delays succession by altering aboveground vegetation and belowground seed banks. PLoS One 9:e91155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson A, Blossey B. 2015. Earthworm invasion, white-tailed deer and seedling establishment in deciduous forests of north-eastern North America. Journal of Ecology 103:153–164. [Google Scholar]
- Drake JA. 1990. The mechanics of community assembly and succession. Journal of Theoretical Biology 147:213–233. [Google Scholar]
- Dufrêne M, Legendre P. 1997. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecological Monographs 67:345–366. [Google Scholar]
- Ehrenfeld JG. 2010. Ecosystem consequences of biological invasions. Annual Review of Ecology, Evolution, and Systematics 41:59–80. [Google Scholar]
- Elton CS. 1958. The ecology of invasions by animals and plants. London: Methuen. [Google Scholar]
- Eschtruth AK, Battles JJ. 2009a. Acceleration of exotic plant invasion in a forested ecosystem by a generalist herbivore. Conservation Biology 23:388–399. [DOI] [PubMed] [Google Scholar]
- Eschtruth AK, Battles JJ. 2009b. Assessing the relative importance of disturbance, herbivory, diversity, and propagule pressure in exotic plant invasion. Ecological Monographs 79:265–280. [Google Scholar]
- Fisichelli NA, Frelich LE, Reich PB, Eisenhauer N. 2013. Linking direct and indirect pathways mediating earthworms, deer, and understory composition in Great Lakes forests. Biological Invasions 15:1057–1066. [Google Scholar]
- Forgione HM. 1993. Limits to the establishment and growth of tree-of-heaven explored (New Jersey). Restoration and Management Notes 11:70–71. [Google Scholar]
- Forsyth DM, Wilson DJ, Easdale T, Kunstler G, Canham CD, Ruscoe WA, Wright EF, Murphy L, Gormley A, Gaxiola A, Coomes DA. 2015. Century scale effects of invasive deer and rodents on the dynamics of forests growing on soils of contrasting fertility. Ecological Monographs 85:157–180. [Google Scholar]
- Fraterrigo JM, Turner MG, Pearson SM. 2006. Interactions between past land use, life-history traits and understory spatial heterogeneity. Landscape Ecology 21:777–790. [Google Scholar]
- Fuller RJ. 2001. Responses of woodland birds to increasing numbers of deer: a review of evidence and mechanisms. Forestry 74:289 –298. [Google Scholar]
- Gibbs RWJ, Hench JE, Hamilton W, D’Loughy K, Hotton D, Hadidian J. 2004. Comprehensive management plan for white-tailed deer in Montgomery County, Maryland. Silver Spring, MD: The Montgomery County Deer Management Work Group; http://www.montgomeryparks.org/PPSD/Natural_Resources_Stewardship/Living_with_wildlife/deer/DeerManagement.shtm (30 June 2016). [Google Scholar]
- Gill RMA, Beardall V. 2001. The impact of deer on woodlands: the effects of browsing and seed dispersal on vegetation structure and composition. Forestry 74:209 –218. [Google Scholar]
- Gleason HA, Cronquist A. 1991. Manual of vascular plants of northeastern United States and adjacent Canada. Bronx, NY: The New York Botanical Garden. [Google Scholar]
- Gleditsch JM, Carlo TA. 2011. Fruit quantity of invasive shrubs predicts the abundance of common native avian frugivores in central Pennsylvania. Diversity and Distributions 17:244–253. [Google Scholar]
- Habeck CW, Schultz AK. 2015. Community-level impacts of white-tailed deer on understorey plants in North American forests: a meta-analysis. AoB PLANTS 7:plv119; doi:10.1093/aobpla/plv119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah L, Lohse D, Hutchinson C, Carr JL, Lankerani A. 1994. A preliminary inventory of human disturbance of world ecosystems. Ambio 23:246–250. [Google Scholar]
- Harper JL. 1977. Population biology of plants. New York: Academic Press. [Google Scholar]
- Heckel CD, Bourg NA, McShea WJ, Kalisz S. 2010. Nonconsumptive effects of a generalist ungulate herbivore drive decline of unpalatable forest herbs. Ecology 91:319–326. [DOI] [PubMed] [Google Scholar]
- Hill MO. 1973. Diversity and evenness: a unifying notation and its consequences. Ecology 54:427–432. [Google Scholar]
- Hobbs NT. 1996. Modification of ecosystems by ungulates. The Journal of Wildlife Management 60:695–713. [Google Scholar]
- Holt RD. 1977. Predation, apparent competition, and the structure of prey communities. Theoretical Population Biology 12:197–229. [DOI] [PubMed] [Google Scholar]
- Hooper DU, Adair EC, Cardinale BJ, Byrnes JEK, Hungate BA, Matulich KL, Gonzalez A, Duffy JE, Gamfeldt L, O’Connor MI. 2012. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486:105–108. [DOI] [PubMed] [Google Scholar]
- Horsley SB, Stout SL, deCalesta DS. 2003. White-tailed deer impact on the vegetation dynamics of a northern hardwood forest. Ecological Applications 13:98–118. [Google Scholar]
- Kalisz S, Spigler RB, Horvitz CC. 2014. In a long-term experimental demography study, excluding ungulates reversed invader’s explosive population growth rate and restored natives. Proceedings of the National Academy of Sciences 111:4501–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane RM, Crawley MJ. 2002. Exotic plant invasions and the enemy release hypothesis. Trends in Ecology and Evolution 17:164–170. [Google Scholar]
- Knapp LB, Canham CD. 2000. Invasion of an old-growth forest in New York by Ailanthus altissima: sapling growth and recruitment in canopy gaps. Journal of the Torrey Botanical Society 127:307–315. [Google Scholar]
- Knapp LB, Fownes JH, Harrington RA. 2008. Variable effects of large mammal herbivory on three non-native versus three native woody plants. Forest Ecology and Management 255:92–98. [Google Scholar]
- Knight TM, Dunn JL, Smith LA, Davis J, Kalisz S. 2009. Deer facilitate invasive plant success in a Pennsylvania forest understory. Natural Areas Journal 29:110–116. [Google Scholar]
- Kocka DM, Steffen DE, Williamson LR. 2000. Deer herd estimation based on catch-per-unit-effort and implications for sharpshooting efficiency. Willmington, NC: Annual Meeting of the Southeast Deer Study Group. [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB. 2013. lmerTest: tests in linear mixed effects models. R package version 2.0-3. https://cran.r-project.org/web/packages/lmerTest/index.html (14 June 2016). [Google Scholar]
- Laliberte AS, Ripple WJ. 2004. Range contractions of North American carnivores and ungulates. BioScience 54:123–138. [Google Scholar]
- Leopold A, Sowls LK, Spencer DL. 1947. A survey of over-populated deer ranges in the United States. The Journal of Wildlife Management 11:162–177. [Google Scholar]
- Levine JM, Adler PB, Yelenik SG. 2004. A meta-analysis of biotic resistance to exotic plant invasions. Ecology Letters 7:975–989. [Google Scholar]
- Levine CR, Winchcombe RJ, Canham CD, Christenson LM, Ronsheim ML. 2012. Deer impacts on seed banks and saplings in eastern New York. Northeastern Naturalist 19:49–66. [Google Scholar]
- Lockwood JL, Hoopes MF, Marchetti MP. 2013. Invasion ecology. West Sussex, UK: John Wiley & Sons. [Google Scholar]
- MacDougall AS, Turkington R. 2005. Are invasive species the drivers or passengers of change in degraded ecosystems?Ecology 86:42–55. [Google Scholar]
- Maron JL, Vilà M. 2001. When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos 95:361–373. [Google Scholar]
- Martin J-L, Baltzinger C. 2011. Interaction among deer browsing, hunting, and tree regeneration. Canadian Journal of Forest Research 32:1254–1264. [Google Scholar]
- McCabe TR, McCabe RE. 1997. Recounting whitetails past. In: McShea WJ, Underwood HB, Rappole JH, eds. The science of overabundance: deer ecology and population management. Washington, DC: Smithsonian Institution Press, 1–26. [Google Scholar]
- McCune B, Grace JB. 2002. Analysis of ecological communities. Gleneden Beach, OR: MjM Software. [Google Scholar]
- McKinney ML, Lockwood JL. 1999. Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends in Ecology & Evolution 14:450–453. [DOI] [PubMed] [Google Scholar]
- McShea WJ. 2000. The influence of acorn crops on annual variation in rodent and bird populations. Ecology 81:228–238. [Google Scholar]
- McShea WJ. 2012. Ecology and management of white-tailed deer in a changing world. Annals of the New York Academy of Sciences 1249:45–56. [DOI] [PubMed] [Google Scholar]
- McShea WJ, Bourg NA. 2008. Assessing the impacts of white-tailed deer and invasive plant interactions on native vegetation at the Potomac Gorge. Front Royal, VA: Smithsonian Institution National Zoological Park Conservation and Research Center. [Google Scholar]
- McShea WJ, Bourg NA. 2009. The impacts of white-tailed deer foraging on woodlots in the Chesapeake and Ohio Canal National Historical Park, and the Antietam and Monocacy National Battlefields. Front Royal, VA: Smithsonian Institution National Zoological Park Conservation and Research Center. [Google Scholar]
- McShea WJ, Bourg NA, Rowan D, Serchan S. 2010. The assessment of impacts of white-tailed deer foraging on woodlots in the Manassas National Battlefield Park. Front Royal, VA: Smithsonian Conservation Biology Institute at the National Zoo. [Google Scholar]
- McShea WJ, Rappole JH. 2000. Managing the abundance and diversity of breeding bird populations through manipulation of deer populations. Conservation Biology 14:1161–1170. [Google Scholar]
- McShea WJ, Underwood BH, Rappole JH. 1997. The science of overabundance: deer ecology and population management. Washington, DC: Smithsonian. [Google Scholar]
- Meiners SJ. 2007. Native and exotic plant species exhibit similar population dynamics during succession. Ecology 88:1098–1104. [DOI] [PubMed] [Google Scholar]
- Mielke PWJ, Berry KJ. 2007. Permutation methods: a distance function approach. New York, NY: Springer-Verlag. [Google Scholar]
- Mosbacher E, Williams C. 2009. Browse preference and browsing intensity of white-tailed deer (Odocoileus virginianus) in Allegheny high plateau riparian forests, USA. Wildlife Biology in Practice 5:11–21. [Google Scholar]
- Myers JA, Vellend M, Gardescu S, Marks PL. 2004. Seed dispersal by white-tailed deer: implications for long-distance dispersal, invasion, and migration of plants in Eastern North America. Oecologia 139:35–44. [DOI] [PubMed] [Google Scholar]
- Newcomb L. 1977. Wildflower guide. Boston, MA: Little, Brown. [Google Scholar]
- Nuttle T, Royo AA, Adams MB, Carson WP. 2013. Historic disturbance regimes promote tree diversity only under low browsing regimes in eastern deciduous forest. Ecological Monographs 83:3–17. [Google Scholar]
- Nuttle T, Yerger EH, Stoleson SH, Ristau TE. 2011. Legacy of top-down herbivore pressure ricochets back up multiple trophic levels in forest canopies over 30 years. Ecosphere 2:1–11. [Google Scholar]
- Nuzzo V, Dávalos A, Blossey B. 2015. Invasive earthworms shape forest seed bank composition. Diversity and Distributions 21:560–570. [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H. 2013. Community ecology package “vegan”. R package version 2.0-10. https://cran.r-project.org/web/packages/vegan/index.html (28 June 2016). [Google Scholar]
- Parker JD, Burkepile DE, Hay ME. 2006. Opposing effects of native and exotic herbivores on plant invasions. Science 311:1459–1461. [DOI] [PubMed] [Google Scholar]
- Parker JD, Hay ME. 2005. Biotic resistance to plant invasions? Native herbivores prefer non-native plants. Ecology Letters 8:959–967. [DOI] [PubMed] [Google Scholar]
- Persson I-L, Danell K, Bergström R. 2000. Disturbance by large herbivores in boreal forests with special reference to moose. Annales Zoologici Fennici 37:251–263. [Google Scholar]
- Putman R, Watson P, Langbein J. 2011. Assessing deer densities and impacts at the appropriate level for management: a review of methodologies for use beyond the site scale. Mammal Review 41:197–219. [Google Scholar]
- Pysřek P, Jarosřík V, Kueera T. 2002. Patterns of invasion in temperate nature reserves. Biological Conservation 104:13–24. [Google Scholar]
- R Development Core Team.. 2014. R: a language and environment for statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rhoads AF, Block TA, Anisko A. 2007. The plants of Pennsylvania: an illustrated manual, 2nd edn. Philadelphia, PA: University of Pennsylvania Press. [Google Scholar]
- Richardson DM, Allsopp N, D’Antonio CM, Milton SJ, Rejmánek M. 2000. Plant invasions – the role of mutualisms. Biological Reviews 75:65–93. [DOI] [PubMed] [Google Scholar]
- Rooney TP. 2001. Deer impacts on forest ecosystems: a North American perspective. Forestry 74:201–208. [Google Scholar]
- Rooney TP, Waller DM. 2003. Direct and indirect effects of white-tailed deer in forest ecosystems. Forest Ecology and Management 181:165–176. [Google Scholar]
- Rooney TP, Wiegmann SM, Rogers DA, Waller DM. 2004. Biotic impoverishment and homogenization in unfragmented forest understory communities. Conservation Biology 18:787–798. [Google Scholar]
- Rossell CR, Patch S, Salmons S. 2007. Effects of deer browsing on native and non-native vegetation in a mixed oak-beech forest on the Atlantic coastal plain. Northeastern Naturalist 14:61–72. [Google Scholar]
- Royo AA, Carson WP. 2006. On the formation of dense understory layers in forests worldwide: consequences and implications for forest dynamics, biodiversity, and succession. Canadian Journal of Forest Research 36:1345–1362. [Google Scholar]
- Royo AA, Collins R, Adams MB, Kirschbaum C, Carson WP. 2010a. Pervasive interactions between ungulate browsers and disturbance regimes promote temperate forest herbaceous diversity. Ecology 91:93–105. [DOI] [PubMed] [Google Scholar]
- Royo AA, Stout SL, deCalesta DS, Pierson TG. 2010b. Restoring forest herb communities through landscape-level deer herd reductions: is recovery limited by legacy effects?Biological Conservation 143:2425–2434. [Google Scholar]
- Russell FL, Zippin DB, Fowler NL. 2001. Effects of white-tailed deer (Odocoileus virginianus) on plants, plant populations and communities: a review. American Midland Naturalist 146:1–26. [Google Scholar]
- Senft R, Coughenour M, Bailey D, Rittenhouse L, Sala O, Swift D. 1987. Large herbivore foraging and ecological hierarchies. BioScience 37:789–799. [Google Scholar]
- Shannon CE. 1948. A mathematical theory of communication. The Bell System Technical Journal 27:379–423. [Google Scholar]
- Sheldon JJ, Causey K. 1974. Deer habitat management–availability use of Japanese honeysuckle by white-tailed deer. Journal of Forestry 72:286–287. [Google Scholar]
- Shelton AL, Henning JA, Schultz P, Clay K. 2014. Effects of abundant white-tailed deer on vegetation, animals, mycorrhizal fungi, and soils. Forest Ecology and Management 320:39–49. [Google Scholar]
- Shen X, Bourg NA, McShea WJ, Turner BL. 2016. Long-term effects of white-tailed deer exclusion on the invasion of exotic plants: a case study in a Mid-Atlantic temperate forest. PLoS One 11:e0151825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SC, Bunker DE, Carson WP. 2006. A null model of exotic plant diversity tested with exotic and native species–area relationships. Ecology Letters 9:136–141. [DOI] [PubMed] [Google Scholar]
- Stohlgren TJ, Binkley D, Chong GW, Kalkhan MA, Schell LD, Bull KA, Otsuki Y, Newman G, Bashkin M, Son Y. 1999. Exotic plant species invade hot spots of native plant diversity. Ecological Monographs 69:25–46. [Google Scholar]
- Strausbaugh PD, Core EL. 1978. Flora of West Virginia, 2nd edn. Granstville, WV: Seneca Books. [Google Scholar]
- Stromayer KAK, Warren RJ. 1997. Are overabundant deer herds in the eastern United States creating alternate stable states in forest plant communities?Wildlife Society Bulletin 25:227–234. [Google Scholar]
- Stromberg JC, Chew MK, Nagler PL, Glenn EP. 2009. Changing perceptions of change: the role of scientists in Tamarix and river management. Restoration Ecology 17:177–186. [Google Scholar]
- Swink F, Wilhelm G. 1994. Plants of the Chicago region, 4th edn. Indianapolis, IN: Indiana Academy of Science. [Google Scholar]
- Turner MG, Gardner RH. 2015. Landscape ecology in theory and practice: pattern and process. New York: Springer. [Google Scholar]
- USDA Forest Service.. 1998. Eastern region invasive plants, ranked by degree of invasiveness as based on information from states. Milwaukee, WI: http://www.fs.fed.us/r9/wildlife/range/weed/?openZSec3B.htm (19 December 2012). [Google Scholar]
- USDA NRCS.. 2012. The plants database. Baton Rouge, LA: National Plant Data Center. [Google Scholar]
- Vavra M, Parks CG, Wisdom MJ. 2007. Biodiversity, exotic plant species, and herbivory: the good, the bad, and the ungulate. Forest Ecology and Management 246:66–72. [Google Scholar]
- Vellend M. 2002. A pest and an invader: white-tailed deer (Odocoileus virginianus Zimm.) as a seed dispersal agent for honeysuckle shrubs (Lonicera L.). Natural Areas Journal 22:230–234. [Google Scholar]
- Vilà M, Espinar JL, Hejda M, Hulme PE, Jarošík V, Maron JL, Pergl J, Schaffner U, Sun Y, Pyšek P. 2011. Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecology Letters 14:702–708. [DOI] [PubMed] [Google Scholar]
- Waller DM, Alverson WS. 1997. The white-tailed deer: a keystone herbivore. Wildlife Society Bulletin 25:217–226. [Google Scholar]
- Webster CR, Jenkins MA, Rock JH. 2005. Biological conservation: long-term response of spring flora to chronic herbivory and deer exclusion in Great Smoky Mountains National Park, USA. Biological Conservation 125:297–307. [Google Scholar]
- White MA. 2012. Long-term effects of deer browsing: composition, structure and productivity in a northeastern Minnesota old-growth forest. Forest Ecology and Management 269:222–228. [Google Scholar]
- Wiegmann SM, Waller DM. 2006. Fifty years of change in northern upland forest understories: identity and traits of “winner” and “loser” plant species. Biological Conservation 129:109–123. [Google Scholar]
- Williams SC, Ward JS, Ramakrishnan U. 2008. Endozoochory by white-tailed deer (Odocoileus virginianus) across a suburban/woodland interface. Forest Ecology and Management 255:940–947. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.