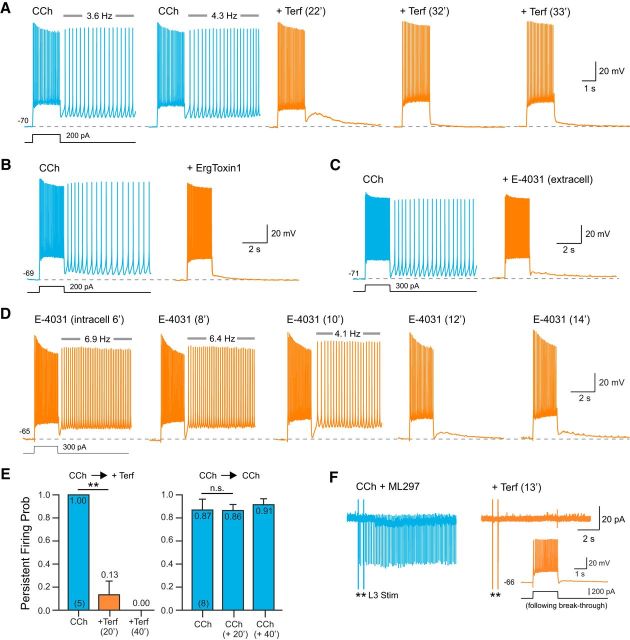
Figure 2.
ERG blockers abolish persistent firing in neocortical neurons. A, Terf (10 μm; orange traces) abolished persistent firing evoked by depolarizing steps in CCh + ML297. Terfenadine exposure time are indicated above each trace. B, The peptide ERG channel blocker ErgToxin1 (50 nm) also abolished persistent firing. C, Extracellular application of E-4031 (10 μm) blocked persistent firing recorded under the same conditions as in A and B. D, Intracellular perfusion with E-4031 (10 μm) abolished persistent firing within 12 min. Persistent firing continued to be evoked by test stimuli in interleaved control experiments without intracellular E-4031 for >40 min (N = 6). E, Left, Plot of the probability of evoking persistent firing >10 s before and after exposure to terfenadine. At 20 min: **p = 0.0029, T = 6.50, df = 4, paired t test; at 40 min: p = 0.0079, Fisher's exact test. Right, Persistent activity could be stably evoked in parallel experiments extending through the same duration without Terf (0 min vs 20 min: p = 0.44; 20 min vs 40 min: p = 0.98; 0 min vs 40 min: p = 0.39, Tukey's HSD test for multiple comparison). F, Terfenadine abolished persistent firing assayed in a cell-attached recording from an L5 neocortical neuron. Response triggered by two extracellular stimuli in L3 (asterisks). Terfenadine abolished the synaptically triggered persistent firing (orange trace). Intracellular recordings from the same neuron following a breakthrough to whole-cell mode demonstrated physiological normal step responses after synaptically evoked persistent firing was abolished (inset).