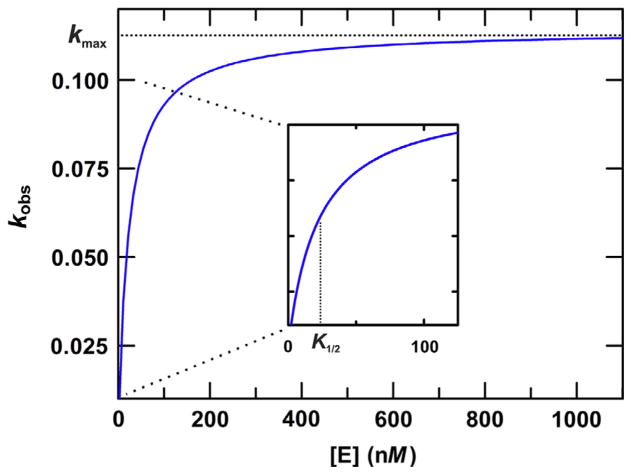
Fig. 3.
Illustration of how kmax is determined by fitting the hyperbolic dependence of kobs on enzyme concentration. A series of single-turnover kinetics experiments are performed using a broad range of enzyme concentrations, including some below and some well above the expected Kd for the E–S complex, and a substrate concentration below that of the lowest enzyme concentration (to satisfy single-turnover conditions). The data are fitted to the following equation: kobs = kmax[E]/([E] + K1/2), where K1/2 is the enzyme concentration for which kobs = kmax/2, which can reflect the Kd for the productive E–S complex. The curve shown has a kmax of 0.113 min−1 and K1/2 of 22 nM.