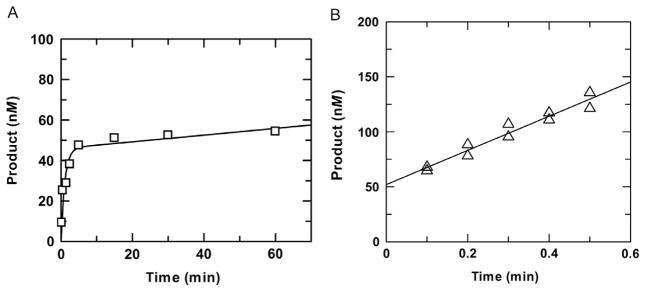
Fig. 4.
Examples of multiple-turnover experiments for which the exponential (burst) phase is (A) or is not (B) observed. (A) Multiple-turnover experiment performed with 0.05μM TDG and a saturating concentration of G·T substrate (1.0μM) at 37°C. Fitting to Eq. (2) gives a rate constant of kobs =0.93±0.29 min−1 and amplitude of A=0.046±0.005 μM for the exponential phase and a rate constant of kcat = 0.0033 ± 0.0025 min−1 for the steady-state phase (using kcat = v/[E]). (B) Multiple-turnover experiment performed with 0.05μM TDG and a saturating concentration of G·5FU substrate (1.5μM). Due to the rapid chemical step for G·5FU substrates, the exponential phase is complete within 1 s and is not observed. A linear fitting of data from two independent experiments gives a steady-state rate constant of kcat = 3.1±0.3 min−1 and y-intercept of 52±4 nM. Close agreement between the amplitude (y-intercept) and the enzyme concentration used in the experiment indicates the enzyme is fully active.