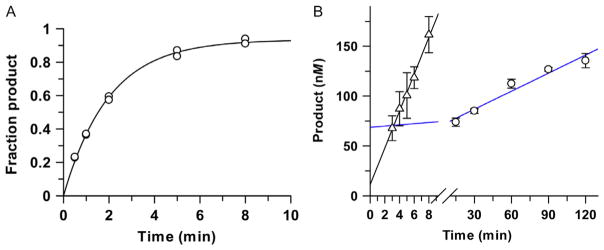
Fig. 5.
Enhancement of TDG steady-state turnover by APE1. (A) Single-turnover experiments, performed with 1.0 μM TDG and 0.3μM G·caC substrate, yield a rate constant of kobs = 0.50 ±0.02 min−1, reflecting the maximal rate of product formation (kmax). (B) Multiple-turnover experiments performed with 0.05 μM TDG and 1.0 μM G·caC substrate give a slow steady-state rate constant of kcat = 0.011 ±0.002 min−1 in the absence of APE1 (blue line, circles). Steady-state turnover is much (32-fold) higher for TDG in the presence of 1.2 μM APE1, (triangles), and approaches the theoretical maximum ( ).