Figure 1. Agonist induced gaps co-localize with baseline gaps.
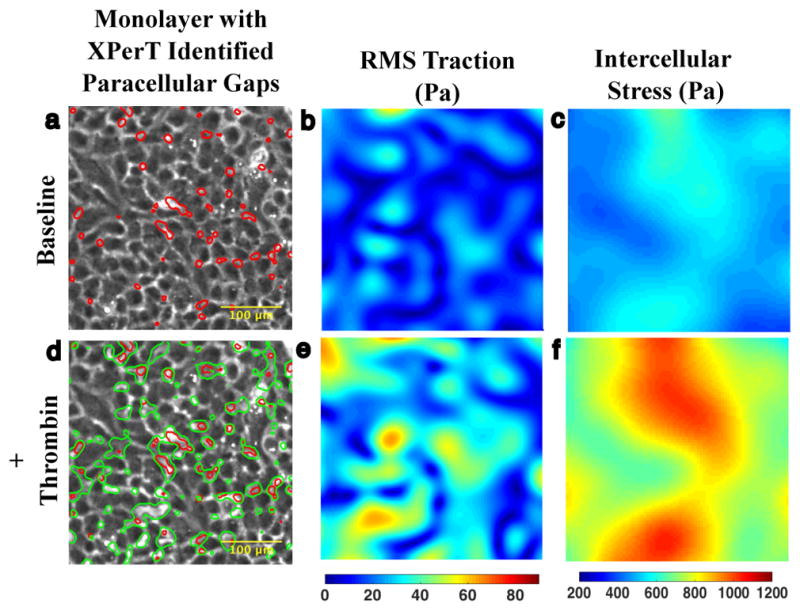
a. 10x phase contrast image of interior of micropatterned monolayer of Human Pulmonary Artery Cells adherent upon a polyacrylamide substrate. At baseline, the monolayer features an array of small XPerT identified paracellular gaps (red). b. The corresponding Fourier Transform Traction Microscopy (FTTM) map of tractions (Pa) is spatially heterogeneous. c. Monolayer Stress Microscopy (MSM) map of intercellular stress (Pa) is also heterogeneous but features larger spatial domains of similar tension. d. 15 minutes after treatment with 0.3 U/ml thrombin the monolayer permeability has increased as evidenced by the larger array of paracellular gaps (green contours). There is substantial overlap between the location of pre- and post-thrombin gaps indicating that much of the increase in permeability has occurred through gap growth rather than de novo gap formation. e. FTTM measured tractions 15 min after thrombin treatment demonstrate an increase in both traction magnitude and heterogeneity. f. MSM map of intercellular stress 15 min after thrombin treatment similarly shows an increase in tension and spatial heterogeneity.
