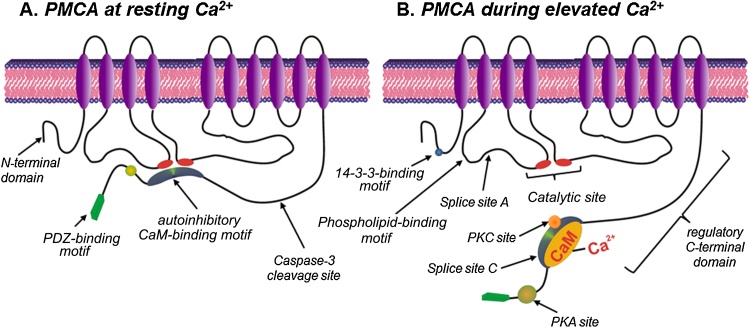
Fig. 1.
Two-dimensional topological model of the structure of the PMCA at low resting [Ca2+]i and following activation at elevated [Ca2+]i. A., at low resting [Ca2+]i the autoinhibitory CaM binding motif within the C-terminal tail of the PMCA associates with the catalytic motif, thereby preventing Ca2+ binding and thus Ca2+ transport. B., when [Ca2+]i is elevated, Ca2+/CaM binds to the autoinhibitory CaM binding motif inducing a conformational change that causes dissociation from the catalytic motif, thereby allowing access to Ca2+ and thus the transport of Ca2+. Additional regulatory motifs include an inhibitory 14-3-3-binding site in the N-terminal region, a stimulatory phospholipid-binding motif in the first cytosolic loop and PKA/PKC phosphorylation consensus sites and a PDZ binding motif in the C-terminal tail. Splice sites A and C can generate splice variants with specific tissue-specific distribution, cellular localization and differential regulation.