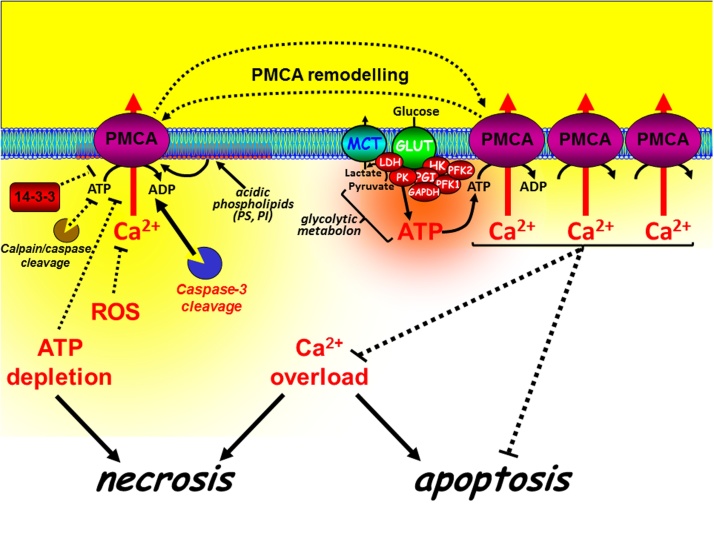
Fig. 3.
Differential regulation and paradoxical role of PMCA in cell death. The PMCA is a high affinity Ca2+ clearance pathway critical for maintaining low resting [Ca2+]i and thus prevention of cytotoxic Ca overload-induced cell death (both necrosis and apoptosis). Inhibition or reduced expression of the PMCA generally increases cell death, whereas activation or overexpression of the PMCA generally inhibits cell death. The PMCA can be directly inhibited by 14-3-3 binding, calpain/caspase cleavage, oxidation by reactive oxygen species (ROS) or by ATP depletion during metabolic stress. However, specific caspase-3 cleavage of the PMCA can remove the autoinihitory CaM-binding domain, resulting in a truncated constitutively active PMCA. Moreover, acidic phospholipids, such as phosphatidylserine (PS) and phosphatidylinositol (PI) can markedly increase the affinity of the PMCA for ATP and Ca2+/CaM, making the PMCA largely insensitive to moderate ATP depletion. Furthermore, glycolytic enzymes can associate with the plasma membrane to form a metabolic complex (metabolon) which provides the PMCA with a privileged ATP supply to the PMCA, even during global ATP depletion. These mechanisms may be important for maintaining survival during severe metabolic stress.