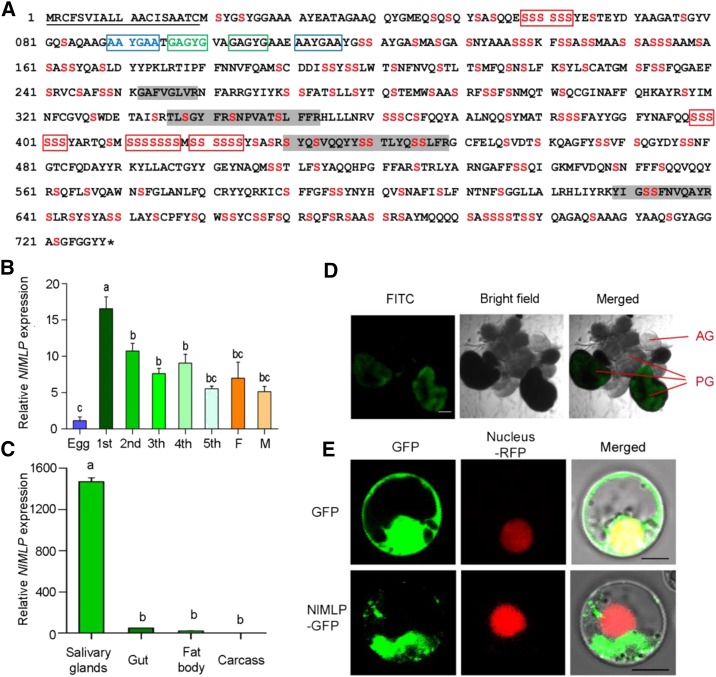
Figure 1.
Molecular characterization of NlMLP. A, Amino acid sequence of NlMLP. The solid underline indicates the signal peptide predicted by SignalP. The asterisk indicates the stop codon. The shaded amino acid residues indicate the peptides detected in BPH-infested rice tissue by liquid chromatography-tandem mass spectrometry analyses. Ser is shown in red. The repeat region is boxed. B and C, Expression patterns of NlMLP at different developmental stages (B) and in different tissues (C). Relative levels of NlMLP expression at the indicated developmental stages (1st to 5th, first to fifth instar; F, female adult; M, male adult) and in different tissues (salivary gland, gut, fat body, and remaining carcass) were normalized against β-actin gene expression, as determined by qRT-PCR. Data represent means ± se of three repeats; n = 30. Different letters above the bars indicate significant differences, as determined by Tukey’s honestly significant difference test (P < 0.05). D, mRNA in situ hybridization of NlMLP in salivary glands. Signals from anti-digoxigenin-fluorescein isothiocyanate (FITC; shown in green) bound to a digoxigenin-labeled antisense riboprobe used for hybridization to NIMLP transcripts were detected by confocal laser-scanning microscopy. AG, Accessory gland; PG, principal gland. Bar = 100 μm. E, NlMLP localizes to the cytoplasm of rice cells when transiently expressed. GFP or NlMLP-GFP fusion protein was expressed in rice protoplasts by polyethylene glycol-mediated transformation. Confocal laser-scanning microscopy was used to investigate fusion protein distribution 16 h after transformation. Bars = 5 μm.