Abstract
The objective of this review was to evaluate the efficacy of selective serotonin reuptake inhibitors (SSRIs) and SSRIs compared with other treatment modalities in preventing relapse after an episode of major depressive disorder (MDD). An Ovid MEDLINE and PsycINFO search (from 1987 to August 2017) was conducted using the following terms: selective serotonin reuptake inhibitors, antidepressants, depression, prevention, prophylaxis, relapse and MDD. Using predefined criteria, two authors independently selected and reached consensus on the included studies. Sixteen articles met the criteria: 10 compared the relapse rate of selective SSRIs with placebo or other SSRIs; one discussed the effectiveness of SSRIs plus psychotherapy, two compared SSRI versus tricyclic antidepressants (TCAs), two were mainly composed of TCAs plus psychotherapy, and one compared SSRIs and serotonin norepinephrine reuptake inhibitors (SNRIs). According to the included studies, the relapse risk in adults was lower when SSRIs were combined with psychotherapy. Results comparing SSRIs and SNRIs were inconclusive. TCAs may be equally as effective as SSRIs. Atypical antidepressants (mirtazapine and St John’s Wort) had no significant difference in efficacy and remission rates compared with SSRIs. Escitalopram appeared to fare better in efficacy than other SSRIs, owing to a higher prophylactic efficacy and lower side effects; however, according to the current data, this difference was not significant. To conclude, this review provides evidence that continuing SSRIs for 1 year reduces risk of MDD and relapse. Furthermore, the combination of SSRIs and cognitive behavioural therapy may effectively reduce relapse. Escitalopram appeared to yield better results and fewer side effects than did other SSRIs or SNRIs. The effectiveness in reducing relapse of SSRIs was similar to that of TCAs and atypical antidepressants.
Keywords: antidepressants, depression, major depression, major depressive disorder, MDD prevention, MDD prophylaxis, specific serotonin reuptake inhibitor, SSRI
Introduction
Major Depressive Disorder (MDD) is the most prevalent of all psychiatric disorders affecting nearly 322 million people or 4.4% of the population worldwide.1 In the US, approximately 16.2% will be affected by depression and this leads to 225 million missed work days and an annual loss of $36.6 billion US dollars.2 It is associated with significant morbidity and mortality, both in adults and children.3,4 Over the next 13 years, depression is projected to be the leading cause of disability in the United States.5 Perhaps the most disabling aspect of depression is the adverse impact in quality of life (QOL), medical comorbidity and mortality.6,7 Depression is commonly treated with antidepressants, psychotherapy, or a combination of both.8 However, low remission rates and high dropout rates are common in these therapies.9–11 MDD has a high risk of relapse, with up to 85% of patients experiencing a recurrence after one MDD episode.12 The presence of residual symptoms after recovery and higher number of previous episodes of depression are strong predictors of recurrence.13–15 Furthermore, among those patients who improve with treatment, the subset who do not achieve clinical remission are at markedly increased risk for relapse.16 Although adjunct therapies may enhance the effects of antidepressant treatments, some studies show few additional benefits.17
Antidepressant therapy with various classes of antidepressants not only treats MDD in the acute phase, but also reduces the risk of relapse and depression recurrence.18,19 Therefore, adherence to antidepressant treatment is important in maintaining remission. However, it is estimated that only 10% of patients with MDD are receiving adequate dosage and duration with antidepressants.12
Although various pharmacological agents are used to treat MDD, selective serotonin reuptake inhibitors (SSRIs) are commonly used as first-line treatment for MDD for adults and children,3,4,20 and various SSRIs are often used for maintenance therapy to prevent relapse.19,20 Other MDD drug agents include: serotonin norepinephrine reuptake inhibitors (SNRIs), norepinephrine-dopamine reuptake inhibitors (NDRIs), tricyclic antidepressants (TCAs), and monoamine oxidase inhibitors (MAOIs).20,21 Numerous studies have shown that psychotherapy, such as cognitive behavior therapy (CBT), with or without pharmacotherapy, is beneficial in reducing the risk of relapse following a depressive episode.18 In understanding relapse risk in MDD, an important finding was that partially remitted patients had higher relapse rates.22 This correlation is very strong, such that residual symptoms in patients could be used as a marker to predict relapse.22,23 Therefore, it is essential that the target in MDD treatment be full remission and therefore, lower the risk of relapse.
Even though some studies have shown the efficacy of SSRI in MDD relapse prevention, a comprehensive review comparing SSRIs with other clinical modalities is lacking. It is currently unclear which of the various antidepressant and CBT treatment options perform better at preventing MDD relapse. Furthermore, there may be differences in efficacy within different antidepressant classes. The purpose of this review is to compare studies focused on SSRIs with other therapies in preventing relapse after an MDD episode. We hope that this review will provide clinicians additional information to help guide them towards optimal treatment modalities for MDD maintenance therapy.
Methods
Search strategy
A systematic literature search was conducted on articles in the Ovid MEDLINE and PsycINFO since the US Food and Drug Administration approval of the first SSRI to the present: from 1987 to August 2017. The keywords used for the search were ‘depress*’ OR ‘major depression’ OR ‘major depressive disorder’, AND prevention OR prophylaxis* OR ‘relapse’, AND ‘SSRI*’ OR ‘specific serotonin re uptake inhibitor*’ AND ‘antidepressants’. The ‘*’ was used to pick up a variation of the search term (e.g. depression or depressive; SSRI or SSRIs) and the quotes are to contain a full search term. This strategy identified 232 relevant articles.
Study selection criteria and methodology
Two authors independently reviewed the abstracts of the 232 articles and selected the articles that met the following inclusion criteria:
articles that were in English or had an availably published English translation;
articles that were published in a peer-reviewed journal;
studies of any design that focused on SSRI use for MDD relapse;
studies that used at least one clear relapse measure.
This narrowed the search results to 24 studies based on the studies’ inclusion criteria. Furthermore, the two authors conducted independently a second review of the full article texts to ensure that the inclusion criteria were met. The two reviewers reached consensus on 16 selected studies to include in the review. The flowchart of the article selection procedure is depicted in Figure 1.
Figure 1.
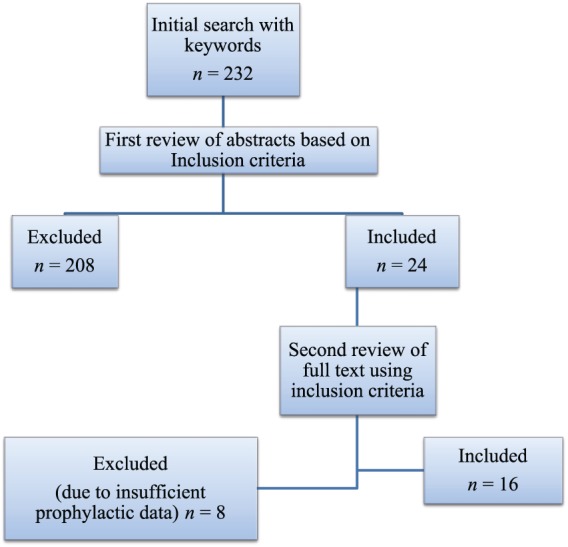
Flowchart of article selection procedure.
Data extraction and yield
Research methodology and key findings in this review are based on the full texts and tables of the selected research articles. Table 1 summarizes the study designs and findings of the selected articles included in this review.
Table 1.
Reference table for included articles.
| Reference [year] |
Antidepressant(s) | Design and population | Time to relapse and measures | Relapse outcomes and other findings | Strengths and limitations |
|---|---|---|---|---|---|
| Doogan and Caillard24 [1992] | Sertraline (50–200 mg/day flexible dosing) mean dose was 69.3–89.2 mg/day | RCT, n = 185 MDD patients; randomized to sertraline or placebo for 44-week maintenance phase |
HAM-D and CGI severity; CGI severity score | 13% of sertraline patients relapsed compared with 45.7% placebo (p < 0.001) | Mean time to relapse was not specified |
| Kamijima et al.25 [2006] | Sertraline (50–100 mg/day flexible dosing) | RCT, n = 117; randomized to either sertraline or placebo for 16-week maintenance phase |
Time to relapse rate curve was significantly higher in sertraline compared with placebo (p = 0.026) HAM-D, CGI-I, MADRS |
8.5% of sertraline patients relapsed; significantly lower relapse rate compared with placebo (19.5%) (p < 0.016) | The time to relapse was not specified |
| Aberg-Wistedt et al.26 [2000] | Sertraline (50–150 mg/day) versus paroxetine (20–40 mg/day) | RCT, n = 353 MDD patients randomized to 24-week treatment | HAM-D, CGI-I, CGI-S, MADRS | Slightly higher rate of relapse in patients with paroxetine (9% versus 2% with sertraline); continuation phase significantly increased remission rates for both SSRIs | The mean time to relapse was not reported |
| Anghelescu et al.12 [2006] | Paroxetine (20–40 mg/day) versus WS®5570 3 × 300 mg/day | RCT multisite study 133 adult MDD outpatients for 160-week maintenance |
WS®5570 (89.5%) and sertraline (92.9%) of patients continued to be treatment responders at end of maintenance phase HAM-D |
WS®5570 was equally effective as paroxetine in preventing relapse after recovery from an episode of moderate-to-severe depression | High attrition rate for both the WS®5570 and paroxetine groups; median time of relapse was not reported |
| Emslie et al.27 [2004] | Fluoxetine versus placebo | RCT Depressed children–adolescents randomized to 36-week fluoxetine or placebo |
Time to relapse was shorter for patients with placebo than fluoxetine (71.2 ± 9.5 days versus 180.7 ± 17.0 days, respectively p = −0.046) measured by CDRS | Fewer relapse rates in fluoxetine patients (34%) versus placebo (60%) | More studies needed to determine predictors of relapse and recurrence |
| Kornstein et al.28 [2006] | Escitalopram (10 or 20 mg/day) | RCT, n = 73 MDD patients | 252 days, 27% relapse rate, measured by MADRS, HAM-D | Escitalopram is effective in reducing recurrence when used as maintenance therapy; discontinuing maintenance with escitalopram and switching to placebo led to depression recurrence even in people with well-controlled depression | |
| Fava et al.29 [1994] | Mainly TCAs (i.e. desipramine, amitriptyline, imipramine, mianserin) ± CBT | RCT n = 40 depressed adults with residual depressive symptoms were randomized to 20-week pharmacotherapy or pharmacotherapy + CBT 2-year follow up |
No significant difference in relapse rates between both groups at 2 year follow up (15% with CBT versus 35% pharmacotherapy only) | Although the relapse rates were insignificantly different at 2 years, CBT group improved residual symptoms more than pharmacotherapy only (p < 0.0001) |
Limitation was the heterogeneous antidepressants used; concomitant use of benzodiazepines |
| Fava et al.30 [1996] | Mainly TCAs (i.e. desipramine, amitriptyline, imipramine, mianserin) ± CBT | RCT n = 40 depressed adults with residual depressive symptoms were randomized to 20-week pharmacotherapy or pharmacotherapy + CBT 4-year follow up |
At 4-year follow up, CBT had lower relapse rate (35% versus 70%) and greater improvement in residual symptoms (p < 0.0001) | Strength was the long-term follow up < 4 years; limitation was the heterogeneous antidepressants used; concomitant use of benzodiazepines |
|
| Gulec et al.22 [2011] | Fluoxetine, mirtazapine, mianserin, amitriptyline, tianeptine | Open-label trial, n = 60 MDD patients for 52-week maintenance phase | A return of index major depressive episode following onset of full or partial remission; score of 18 or more on HAMD-17 for at least 2 consecutive weeks in partial or full remission. | Relapse rate at week 52 was 8.33% for full remitters and 25% for partial remitters (threefold); partially remitted patients had higher relapse rate as compared with fully remitted patients having depression |
Focus was the relapse rate on partially versus full remitters; many concomitant medications (stimulants, benzodiazepines and antipyschotics) were coprescribed; maintenance phase drug treatments were unspecified |
| Dotoli et al.13 [2006] | Fluvoxamine SSRI | Maintenance phase treatment study, n = 101 remitted patients (80 unipolar, 21 bipolar) during 6-month follow up. |
Relapsed patients had a longer mean duration of index depressive episodes than nonrelapsed patients (23.3 ± 24.2 versus 17.2 ± 17.3 weeks) measured by SASS |
8.9% relapsed within first 2 months of continuation | Median time to relapse was not compared between fluvoxamine, no placebo control |
| Peselow et al.18 [2015] | SSRIs versus SSRIs and SSRI versus (fluoxetine, escitalopram, sertraline, paroxetine) ± CBT | Naturalistic long-term study, n = 387 patients who had remitted or responded with SSRIs | Escitalopram had highest prophylactic efficacy or prevention of depressive episodes (36%), fluoxetine (33.3%), sertraline (21.3%), paroxetine (12.85), but ns between SSRIs MADRS |
41% SSRI + CBT were episode free compared with 18% of patients in the SSRI-only group who maintained remission (p < 0.05) | Small size of CBT group |
| Pundiak et al.3 [2008] | Fluoxetine, paroxetine, sertraline | Naturalistic long-term study, n = 60 MDD patients, entered into 1-month continuation phase followed by a 5-year maintenance phase with SSRI monotherapy and assessed every 3 months for mood symptoms | Median time of relapse with SSRI was 38 months, exceeding the 10-month time of patients who had discontinued | Continuation of SSRIs had better probability of maintaining remission during the first year than with discontinuation [Survival probability 0.70 versus 0.40]) significant difference for over 30 months | Major strength was the long term follow up. Community, pragmatic setting Limitation was the lack of separating analyses based on the SSRI (fluoxetine versus paroxetine versus sertraline) |
| Kaymaz et al.19 [2008] | SSRIs (fluoxetine, sertraline, citalopram, paroxetine) and TCAs (amitriptyline, imipramine, nortriptyline, maprotiline, dothiepin) | Meta-analysis of RCTs (30 trials with 4890 participating patients) | Reduction of relapse risk was significant for SSRIs: (OR = 0.24, Cl 0.20–0.29), TCAs: (OR = 0.29, Cl 0.23–0.38) at 1-year follow up CGI-I, CGI-S, MADRS, HAM-D, GAS, MINI, Raskin depression scale, RDC, SCID-P |
Antidepressants are effective in reducing relapse risk in maintenance phase; however, no significant difference seen between SSRIs and TCAs | Direct comparison between TCA and SSRI; limitation was that neither the time to relapse nor the mean relapse rate for both groups was reported |
| Bauer et al.31 [2009] | Venlafaxine versus sertraline, citalopram, escitalopram and placebo | Meta-analysis of RCT trials up to 2007 based on venlafaxine trials with MDD patients | HAM-D, BDI, MADRS | Long-term treatment with venlafaxine was effective at reducing relapse after major depressive episode (OR = 0.37, Cl 0.27–0.51) compared with placebo; venlafaxine was more effective than SSRIs and as effective as TCA in treating major depressive episode via remission and response rates. | Wide CIs were due to the heterogeneity of the analyzed clinical trials |
| Garnock-Jones et al.20 [2010] | Escitalopram (10 or 20 mg/day) | Meta-analysis of three RCTs, n = 311 MDD patients randomized to maintenance phase of escitalopram or placebo for 24–52 weeks. | Time of relapse was significantly longer in escitalopram than placebo (p < 0.05) MADRS and HAM-D | Escitalopram effectively prevented relapse in adults who had previously responded to escitalopram | One of the three studies analyzed did not report the number of relapsed patients (calculated here ~14 patients or 9% relapse rate) |
| Yang et al.23 [2010] | Fluoxetine ⩾ 40 mg/day |
Post hoc analysis, n = 262 MDD patients who responded to fluoxetine by week 12 (131 continued fluoxetine, 131 switched to placebo) |
Based on time to relapse as a dependent variable, the hazard ratio = 1.55 or 2.4; symptom questionnaire (SQ) and 90-item self-report Hopkins symptom checklist (SCL-90) | Fluoxetine relapse rate at 6 months was 35.2% and 61.8% for placebo; at 1 year, 45.9% for fluoxetine and 72% for placebo | Time to relapse was a dependent variable in a prediction model but the mean time to relapse was not described |
RCT, randomized controlled trial; MDD, major depressive disorder; HAM-D, Hamilton Depression Rating Scale; CGI, Clinical Global Impression Scale; CGI-S, Clinical Global Impression Severity Scale; SSRIs, selective serotonin reuptake inhibitors; SASS, Social Adaptation Self-Evaluation Scale; GAS, Global Assessment Scale; MINI, Mini International Neuropsychiatric Interview; MADRS, Montgomery-Asberg Depression Rating Scale; WS®5570, Hypericum extract; CDRS, Children’s Depression Rating Scale; CBT, cognitive behavior therapy; TCAs, tricyclic antidepressants; ns, not significant; OR, odds ratio; CI, confidence interval; RDC, Research Diagnostic Criteria; SCID-P, Structured Clinical Interview D for DSM-IV - Patient Version; BDI, Beck Depression Inventory.
Results
The results of the review findings are depicted in Table 1.
Efficacy of selective serotonin reuptake inhibitors for maintenance of major depressive disorder
There were 10 studies that specified relapse rates or relapse risk ratios among SSRIs. SSRIs are common first-line agents for initial treatment and prophylaxis of MDD, and it is important to consider the ability to acutely treat an episode and prevent future depression episodes. In regards to sertraline (50–200 mg/day), the relapse rate was 8.5% versus 19.5% placebo in a 44-week maintenance trial24 and 13% versus 45.7% placebo in a 16-week maintenance trial.25 In comparing sertraline with another SSRI, sertraline had a slightly lower relapse rate of 2% versus 9% with paroxetine (20–40 mg/day) in a randomized clinical trial (RCT) of 24-week maintenance treatment.26 Although significance was not specified for this difference, the remission rates significantly increased with both SSRIs. The relapse rate of paroxetine was directly compared with Hypericum extract (WS®5570) in a 160-week maintenance treatment multisite study, it was determined that both SSRIs were equally as effective at preventing relapse from an episode of moderate to severe depression.12 In a post hoc analysis, Yang and colleagues determined that adult MDD patients on fluoxetine had a lower relapse rate (45.9%) than the placebo group (72%) after a 1-year follow up.23 Among depressed children and adolescents, the relapse rate with fluoxetine was significantly lower in an RCT at 34% versus 60% placebo at 36-week follow up.27 Similar results were shown in two other meta-analyses of escitalopram, sertraline, citalopram and paroxetine, all showing statistically significant decreases in relapse rates when compared with placebo.19,20 In comparing SSRIs for preventing depressive episodes, four SSRIs were compared in a naturalistic long-term study. Escitalopram was the most effective at preventing depressive episodes, with a prophylactic efficacy of 36%, followed by fluoxetine (33.3%), then by sertraline (21.3%) and lastly by paroxetine (12.85%). Although these values appear to greatly differ, there was no significant difference among the SSRIs, as measured by a change in Montgomery-Asberg Depression Rating Scale (MADRS) score.18 In a naturalistic study, the continuation of SSRIs significantly had better probability at maintaining remission within the first year when compared with discontinuation of SSRIs (0.70 versus 0.40).3 Despite SSRIs’ role as the first-line MDD treatment, a large-scale clinical trial directly comparing SSRIs with other treatment options such as SNRIs, TCAs, atypical antidepressants and CBT is warranted. Lastly, in a 6-month maintenance phase of treatment with fluvoxamine, only 8.9% of patients relapsed within the first 2 months of the phase. However, significance cannot be assessed, since the relapse rate for placebo was not reported.13
Selective serotonin reuptake inhibitors versus serotonin norepinephrine reuptake inhibitors for major depressive disorder
SNRIs, such as venlafaxine, were compared with SSRIs in one study from our search results. Bauer and colleagues31 compiled a meta-analysis of 27 studies in order to establish the efficacy of venlafaxine compared with SSRIs. Venlafaxine was found to produce more favorable outcomes when compared with imipramine, clomipramine, amitriptyline, dothiepin, amineptine and maprotiline with an odds ratio (OR) of 1.22 [95% confidence interval (CI) 0.96–1.54]. The relapse rate with venlafaxine was effective at reducing relapse after a major depressive episode (OR = 0.37, CI 0.27–0.51), compared with placebo. However, the relapse rates for SSRIs were not calculated; therefore, the relapse rates could not be compared between SSRI and SNRIs. Studies on other SNRIs such as duloxetine found that SSRI use produced a more significant decrease in the MADRS total score.20 Garnock-Jones and colleagues noted that escitalopram achieved a faster short-term response than venlafaxine, making escitalopram more desirable for acute and severe MDD.20 Although, dropout rates in pooled studies did not show an appreciable difference between venlafaxine versus SSRI treatment (95% CI 0.95–1.19; p = 0.26), it was shown that the incidence of side effects in the venlafaxine trials was higher by a statistically relevant margin with an OR of 1.45 (95% CI 1.23–1.70; p < 0.001).27 Some of these side effects included dry mouth, insomnia, nausea, and vomiting. In addition, venlafaxine was associated with increased suicide risk4,20 compared with the low OR for risk of suicide and suicidal thoughts with most SSRIs.32 Despite the side effect profile and depending on the clinical and patient preference, data suggest that venlafaxine could be the favorable choice for relapse prevention.
Selective serotonin reuptake inhibitors versus tricyclic antidepressants as maintenance therapy for major depressive disorder
There were four articles that reported the relapse rates of TCAs, while only one study directly compared the relapse risk between TCAs versus SSRIs. In a meta-analysis of 30 RCTs (n = 4890 patients), the reduction of relapse risk was significant for TCAs, with OR = 0.29 (Cl 0.23–0.38) at 1-year follow up. The OR was similar for SSRIs (OR = 0.24, Cl 0.20–0.29). Although the OR was greater for TCAs than for SSRIs, there was no statistical significance between the two values. In addition, the CI was greater for TCAs, given the heterogeneity of the RCTs.19 In a pragmatic open-label trial, the relapse rates were reported for all antidepressants (including fluoxetine, mirtazapine, mianserin, amitriptyline and tianeptine) and compared with the full versus partial remitters. At week 52, the relapse rate was 8.33% for full remitters and significantly higher for partial remitters (25%). One weakness of the study was the lack of subanalysis according to antidepressant type.22 In studies by Fava and colleagues, patients were randomly assigned to two groups; either antidepressants or antidepressants + CBT. In these studies, the antidepressants included various drug classes; however, roughly 50% were TCAs. The relapse rates were lower with the combination at 2- and 4-year follow up.29,30 Given the CBT component, these results will be described subsequently.
Other alternatives to selective serotonin reuptake inhibitors for maintenance therapy for major depressive disorder
An alternative to typical antidepressants is Hypericum extract WS®5570, also known as St John’s Wort, which has minimal side effects. At a dose of WS®5570 3 × 300 mg/day, the relapse rate did not significantly differ to paroxetine (20 or 40 mg/day). Although more studies are warranted to determine clinical utility of WS®5570, existing research revealed that it may be a clinically relevant antidepressant with a favorable side-effect profile.
Cognitive behavior therapy as an adjuvant to selective serotonin reuptake inhibitors for maintenance of major depressive disorder
CBT is a desirable nonpharmacotherapeutic option that can be used in addition to or in place of SSRI therapy for patients that are wary of pharmacotherapy when treating MDD in the acute and maintenance phases. In a 34-month naturalistic prospective study, Peselow and colleagues analyzed the utility of using CBT as an adjunct therapy for patients currently taking SSRI. Relative to patients in the SSRI group, time to MDD recurrence was longer and the remission maintenance rate was higher with the SSRI + CBT group (41%), whereas only 18% of patients in the SSRI-only group maintained remission.18 A separate analysis by Fava and colleagues showed no significant difference in the maintenance therapy groups using either pharmacotherapy only or CBT + pharmacotherapy at a 2-year follow up.29 However, when the follow up was extended to 4 and then 6 years, the long-term benefits of CBT became clearer with fewer relapses (35% and 70%; p < 0.05, respectively) and fewer new episodes of depression (mean 0.8 ± 0.95 versus 1.7 ± 1.3; p < 0.05) than the SSRI group.30 In adolescents and children, psychotherapy has not been shown to be as effective as pharmacotherapy; especially among MDD with comorbid diagnoses. Thus, CBT does not seem to be adequate as a standalone therapy in pediatric MDD.4
Discussion
When choosing an antidepressant for the treatment of MDD, it is important not only to consider its effectiveness in treating acute episodes but also in the prevention of future episodes. Three meta-analysis reviews suggested that continuing treatment with SSRIs reduces the risk of experiencing another episode of depression (relapse) and may reduce the risk of experiencing another episode within the following year (recurrence). Escitalopram appeared to have a higher prophylactic efficacy; however, differences between other SSRIs were found to be insignificant.18
One study compared the efficacy in short-term response and remission of MDD between a SNRI (venlafaxine) and SSRIs and found that venlafaxine was more effective than SSRIs, with higher remission and response rates.31 While this is compelling evidence for the efficacy of venlafaxine, it should be noted that a review article by Sheehan and colleagues found venlafaxine and SSRIs to be equally as effective.33 This contrasts to a review by Garnock-Jones and colleagues that concluded SSRIs achieved a more rapid short-term response than venlafaxine.20 Of interest, the efficacy of venlafaxine at reducing relapse is superior to placebo. However, the efficacy of reducing relapse between venlafaxine and other antidepressants was not assessed. Therefore, no conclusive treatment recommendations can be made on the basis of this review. There is evidence that TCAs may be more effective than SSRIs in reducing relapse. However, these differences appear to be insignificant.19 Additionally, TCA side effects would limit their use to patients who cannot tolerate SSRIs.
The adjunct of psychotherapy appears to be beneficial in preventing future depression episodes among adults.18 Augmentation of CBT was found to be significantly associated with fewer relapses (35% versus 70%; p < 0.05) and fewer new episodes (recurrences) of depression than the SSRI-only group at a 4- and 6-year follow up.29,30 In adolescents and children, psychotherapy has not been shown to be as effective as pharmacotherapy; especially among MDD patients with comorbid diagnoses. Thus, CBT does not seem to be adequate as a standalone therapy in pediatric MDD.4 Given these data, there are several treatment options patients can consider. For patients who wish to avoid pharmacotherapy and are financially able and willing to commit to long-term CBT, there is therapeutic benefit without the risk of side effects. However, patients who wish to alleviate their MDD maximally can opt to take advantage of the apparent synergism available when using a combined SSRI and CBT therapy. Finally, for patients unable to commit to CBT, SSRIs offer a more effective alternative to CBT.
This review has further confirmed that no one drug fits all, and it is crucial to determine the antidepressant that is tailored to the patient’s needs and considering the individual risk/benefit ratio. Additionally, genetic screening may show promise in maximizing therapeutic benefits of antidepressants without undergoing trial-and-error methods. However, Dotoli and colleagues failed to find any genetic markers that correlated with SSRI response.13 Future research of MDD treatment should be aimed at finding ways to customize comprehensive treatment for patients. Traditionally, the goal of antidepressant therapy has been to improve symptoms or achieve response (50% reduction in symptom severity). However, research findings support the need to reach full symptomatic remission (minimal or no symptoms) since lingering of residual symptoms could serve as an obstacle in achieving recovery. More importantly, improvements in functioning and QOL have emerged not only as optimal therapeutic goals but also essential prominent steps in recovery.36 Therefore, it is crucial to comprehensively treat depression with a battery of tools: pharmacotherapy and augmentation with psychotherapy, actively seeking to improve QOL and functioning.
Conclusion
This review article analyzed the available literature on the effectiveness of SSRIs in preventing MDD relapse, in comparison with alternative therapies, showing some evidence that continuing SSRIs for 1 year appears to reduce risk of MDD and relapse. Escitalopram appeared to yield better results and fewer side effects. The efficacy of SSRIs was similar to that of TCAs and atypical antidepressants (mirtazapine, St John’s Wort). Future combination trials comparing antidepressants and psychotherapy (e.g. CBT) are needed to guide treatment decisions to achieve remission, improve functioning and QOL, leading to eventual full recovery.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Steven S. Clevenger, Western University of Health Sciences College of Osteopathic Medicine, and Cedars-Sinai Medical Center Department of Psychiatry, Los Angeles, CA, USA
Devvrat Malhotra, Western University of Health Sciences College of Osteopathic Medicine, and Cedars-Sinai Medical Center Department of Psychiatry, Los Angeles, CA, USA.
Jonathan Dang, Cedars-Sinai Medical Center Department of Psychiatry, Los Angles, CA, USA.
Brigitte Vanle, Cedars-Sinai Medical Center Department of Psychiatry, Los Angles, CA, USA.
Waguih William IsHak, Cedars-Sinai Medical Center Department of Psychiatry and UCLA, 8730 Alden Drive, Thalians E-132, Los Angeles, CA 90048-0750, USA.
References
- 1. World Health Organization (WHO). Depression and other common mental disorders: global health estimates, http://apps.who.int/iris/bitstream/10665/254610/1/WHO-MSD-MER-2017.2-eng.pdf (accessed 27 August 2017).
- 2. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62: 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pundiak TM, Case BG, Peselow ED, et al. Discontinuation of maintenance selective serotonin reuptake inhibitor monotherapy after 5 years of stable response: a naturalistic study. J Clin Psychiatry 2008; 69: 1811–1817. [DOI] [PubMed] [Google Scholar]
- 4. Emslie GJ, Mayes TL, Ruberu M. Continuation and maintenance therapy of early-onset major depressive disorder. Paediatr Drugs 2005; 7: 203–217. [DOI] [PubMed] [Google Scholar]
- 5. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006; 3: e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. IsHak WW, Greenberg JM, Balayan K, et al. Quality of life: the ultimate outcome measure of interventions in major depressive disorder. Harv Rev Psychiatry 2011; 19: 229–239. [DOI] [PubMed] [Google Scholar]
- 7. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med 2013; 10: e1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spijker J, van Straten A, Bockting CL, et al. Psychotherapy, antidepressants, and their combination for chronic major depressive disorder: a systematic review. Can J Psychiatry 2013; 58: 386–392. [DOI] [PubMed] [Google Scholar]
- 9. Mathew SJ, Charney DS. Publication bias and the efficacy of antidepressants. Am J Psychiatry 2009; 166: 140–145. [DOI] [PubMed] [Google Scholar]
- 10. Pigott HE, Leventhal AM, Alter GS, et al. Efficacy and effectiveness of antidepressants: current status of research. Psychother Psychosom 2010; 79: 267–279. [DOI] [PubMed] [Google Scholar]
- 11. Turner EH, Matthews AM, Linardatos E, et al. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med 2008; 358: 252–260. [DOI] [PubMed] [Google Scholar]
- 12. Anghelescu IG, Kohnen R, Szegedi A, et al. Comparison of Hypericum extract WS 5570 and paroxetine in ongoing treatment after recovery from an episode of moderate to severe depression: results from a randomized multicenter study. Pharmacopsychiatry 2006; 39: 213–219. [DOI] [PubMed] [Google Scholar]
- 13. Dotoli D, Spagnolo C, Bongiorno F, et al. Relapse during a 6-month continuation treatment with fluvoxamine in an Italian population: the role of clinical, psychosocial and genetic variables. Prog Neuropsychopharmacol Biol Psychiatry 2006; 30: 442–448. [DOI] [PubMed] [Google Scholar]
- 14. Nierenberg AA, Husain MM, Trivedi MH, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med 2010; 40: 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hardeveld F, Spijker J, De Graaf R, et al. Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatr Scand 2010; 122: 184–191. [DOI] [PubMed] [Google Scholar]
- 16. Paykel ES. Partial remission, residual symptoms, and relapse in depression. Dialogues Clin Neurosci 2008; 10: 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Warden D, Rush AJ, Trivedi MH, et al. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep 2007; 9: 449–459. [DOI] [PubMed] [Google Scholar]
- 18. Peselow ED, Tobia G, Karamians R, et al. Prophylactic efficacy of fluoxetine, escitalopram, sertraline, paroxetine, and concomitant psychotherapy in major depressive disorder: outcome after long-term follow-up. Psychiatry Res 2015; 225: 680–686. [DOI] [PubMed] [Google Scholar]
- 19. Kaymaz N, van Os J, Loonen AJ, et al. Evidence that patients with single versus recurrent depressive episodes are differentially sensitive to treatment discontinuation: a meta-analysis of placebo-controlled randomized trials. J Clin Psychiatry 2008; 69: 1423–1436. [DOI] [PubMed] [Google Scholar]
- 20. Garnock-Jones KP, McCormack PL. Escitalopram: a review of its use in the management of major depressive disorder in adults. CNS Drugs 2010; 24: 769–796. [DOI] [PubMed] [Google Scholar]
- 21. Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet (London, England) 2009; 373: 746–758. [DOI] [PubMed] [Google Scholar]
- 22. Gulec M, Selvi Y, Boysan M, et al. Ongoing or re-emerging subjective insomnia symptoms after full/partial remission or recovery of major depressive disorder mainly with the selective serotonin reuptake inhibitors and risk of relapse or recurrence: a 52-week follow-up study. J Affect Disord 2011; 134: 257–265. [DOI] [PubMed] [Google Scholar]
- 23. Yang H, Chuzi S, Sinicropi-Yao L, et al. Type of residual symptom and risk of relapse during the continuation/maintenance phase treatment of major depressive disorder with the selective serotonin reuptake inhibitor fluoxetine. Eur Arch Psychiatry Clin Neurosci 2010; 260: 145–150. [DOI] [PubMed] [Google Scholar]
- 24. Doogan DP, Caillard V. Sertraline in the prevention of depression. Br J Psychiatry 1992; 160: 217–222. [DOI] [PubMed] [Google Scholar]
- 25. Kamijima K, Burt T, Cohen G, et al. A placebo-controlled, randomized withdrawal study of sertraline for major depressive disorder in Japan. Int Clin Psychopharmacol 2006; 21: 1–9. [DOI] [PubMed] [Google Scholar]
- 26. Aberg-Wistedt A, Agren H, Ekselius L, et al. Sertraline versus paroxetine in major depression: clinical outcome after six months of continuous therapy. J Clin Psychopharmacol 2000; 20: 645–652. [DOI] [PubMed] [Google Scholar]
- 27. Emslie GJ, Heiligenstein JH, Hoog SL, et al. Fluoxetine treatment for prevention of relapse of depression in children and adolescents: a double-blind, placebo-controlled study. J Am Acad Child Adolesc Psychiatry 2004; 43: 1397–1405. [DOI] [PubMed] [Google Scholar]
- 28. Kornstein SG, Bose A, Li D, et al. Escitalopram maintenance treatment for prevention of recurrent depression: a randomized, placebo-controlled trial. J Clin Psychiatry 2006; 67: 1767–1775. [DOI] [PubMed] [Google Scholar]
- 29. Fava GA, Grandi S, Zielezny M, et al. Cognitive behavioral treatment of residual symptoms in primary major depressive disorder. Am J Psychiatry 1994; 151: 1295–1299. [DOI] [PubMed] [Google Scholar]
- 30. Fava GA, Grandi S, Zielezny M, et al. Four-year outcome for cognitive behavioral treatment of residual symptoms in major depression. Am J Psychiatry 1996; 153: 945–947. [DOI] [PubMed] [Google Scholar]
- 31. Bauer M, Tharmanathan P, Volz HP, et al. The effect of venlafaxine compared with other antidepressants and placebo in the treatment of major depression: a meta-analysis. Eur Arch Psychiatry Clin Neurosci 2009; 259: 172–185. [DOI] [PubMed] [Google Scholar]
- 32. Gunnell D, Saperia J, Ashby D. Selective serotonin reuptake inhibitors (SSRIs) and suicide in adults: meta-analysis of drug company data from placebo controlled, randomised controlled trials submitted to the MHRA’s safety review. BMJ 2005; 330: 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sheehan DV, Kamijima K. An evidence-based review of the clinical use of sertraline in mood and anxiety disorders. Int Clin Psychopharmacol 2009; 24: 43–60. [DOI] [PubMed] [Google Scholar]


