Abstract
We evaluated the use of oral deformities as reliable proxies for determining Batrachochytrium dendrobatidis (Bd) infection in tadpoles of six anuran species of the Atlantic Forest in southeastern Brazil. We examined oral discs of 2156 tadpoles of six species of anurans collected in 2016: Aplastodiscus albosignatus, Boana albopunctata, Boana faber, Scinax hayii, Crossodactylus caramaschii, and Physalaemus cuvieri. Three oral deformities were recognized: lack of keratinization only in upper and/or lower jaw sheaths, lack of keratinization only in upper or lower tooth rows, and both deformities together. A subsample composed of all the individuals possessing oral deformities (N = 195) plus randomly selected individuals without oral deformities (N = 184) were tested for Bd via qPCR. Oral deformities were observed in all six species, but only five were infected with Bd. Since we found that dekeratinization of tooth rows was not associated with the presence of Bd in any of the studied species we used a new proxy (jaw sheaths dekeratinization with or without dekeratinization in tooth rows: JSD-proxy) for Bd detection. Our results showed a nonrandom relationship between Bd infection and JSD-proxy in three species of the family Hylidae. However, the use of JSD-proxy for Bd detection in these species resulted in up to 30.8% false positives and up to 29.3% false negatives. The use of the JSD-proxy in species for which no relationship was found reached 100% of false positives. We conclude that the use of oral dekeratinization as a generalized proxy for Bd detection in tadpoles should not be used as a single diagnosis technique.
Introduction
Global loss of biodiversity is one of the most serious problems of our time, with Amphibia being the most affected vertebrate class [1, 2]. Habitat loss, introduction of exotic species, environmental contamination, increased UV radiation, climate change and infectious diseases have been identified as major causes of amphibian population declines [3–17]. Chytridiomycosis, an infectious fungal disease caused by Batrachochytrium dendrobatidis (Bd) [15], has been linked to many incidents of amphibian mass mortality worldwide [15,18,19], and is considered one of the greatest causes of global amphibian declines [20]. In amphibians, Bd infection occurs only in keratinized tissues, which are restricted to the oral region (jaw sheaths and teeth) of tadpoles, and the epidermis of metamorphs and adults [15, 21–24].
Several studies have established a relationship between Bd infection and the occurrence of anomalies in the oral region of tadpoles of a number of different amphibian species. Fellers et al. [25] found that 67% of Rana muscosa tadpoles with abnormally keratinized mouthparts were infected by Bd. In turn, Knapp and Morgan [26] found for the same species that 89% of tadpoles with less than 90% jaw sheath pigmentation were infected. This relationship was later reinforced by Drake et al. [27], who found clear nonrandom relationships between oral deformities and Bd presence in Lithobates sphenocephalus (= Rana sphenocephala) tadpoles. A similar result was reported for Hylodes japi tadpoles, for which 94.5% of infected individuals possessed depigmented mouthparts [28]. These results suggest the possibility of using tadpole oral deformities as a general proxy for Bd detection in tadpoles, as has been previously suggested by Fellers et al. [25] and recently applied by Carvalho et al. [29].
Despite this promising proxy for which only a quick visual inspection is required, there are many studies that have indicated that oral deformities are not always related to Bd infection. For example, no relationship was found between depigmentation of mouthparts and Bd infection of Lithobates catesbeianus (= Rana catesbeiana) and Pseudacris regilla [30]. Furthermore, a laboratory study found no differences in the proportions of tadpoles with mouthpart abnormalities between Bd infected and uninfected individuals of Anaxyrus boreas (= Bufo boreas) and Pseudacris regilla (= Hyla regilla) [31]. In addition, many other factors have been associated with oral deformities in tadpoles, including low temperatures [32], water contamination [33], nutrition [21], competition [34], and predation risk [35]. Herein we quantitatively evaluate, in detail, the use of oral deformities as a reliable proxy for determination of Bd infection in six anuran species from southeastern Brazil.
Material and methods
Ethics statement
Field studies did not involve endangered or protected species.
Tadpoles were captured in accordance with collection permits, and subsequently killed using lidocaine, and preserved in 90% alcohol for subsequent study of oral deformities. Collection permits were provided by Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) (#47148–2). All sampling procedures were reviewed and specifically approved by ICMBio and Comissão Técnico-Científica do Instituto Florestal (COTEC; a committee of Instituto Florestal, the state research agency and responsible for the reserve) (Processo SMA #260108–001.809/2015).
Study animals
A total of 2156 tadpoles were studied. External morphology and tooth rows formulae were used to identify the species of tadpoles. Tadpoles were collected by dip netting during February (2016) in Núcleo Curucutu (23o 59’ 08.52”S, 46o 44’ 35.76” W), Parque Estadual da Serra do Mar (São Paulo State, Brazil), an understudied old growth area of Atlantic Forest in southeastern Brazil [36].
Identification of oral deformities
It is important to emphasize that studies regarding the relationship between Bd infection and oral deformities have used different, and sometimes ambiguous terms for describing the deformities. For example, while some of studies describe oral deformities as depigmentation of mouthparts (e.g. [28, 30]), others refer to them as dekeratinization (e.g. [27, 31]). This ambiguity was recognized by Altig [37], who pointed out that the “depigmentation” as described in some studies was, in fact, dekeratinization of mouthparts. Therefore, we have chosen to use the term dekeratinization of mouthparts.
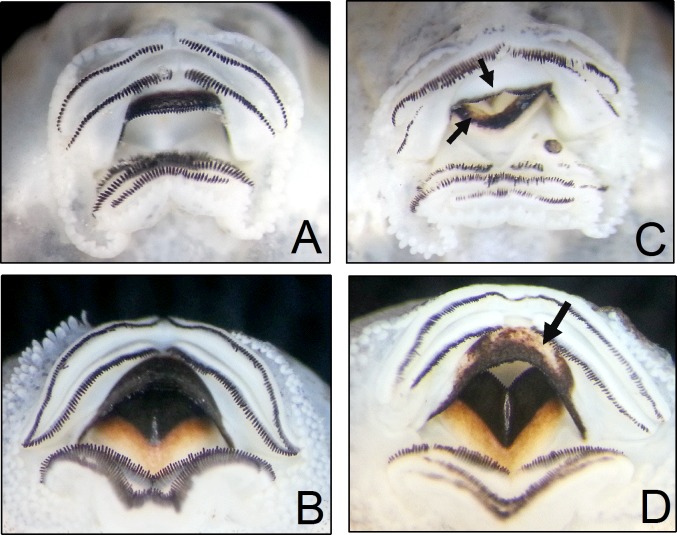
All individuals studied were between Gosner stages 25 and 40, since the oral disc of tadpoles within these stages normally possesses completely keratinized tissues [24, 38]. Oral discs of tadpoles were examined in detail using a stereoscopic microscope (Leica 54 MZ75), and tadpoles were classified in accordance with dekeratinization (from partial to complete absence of keratinized structures) that presented in mouthparts: JS (tadpoles with lack of keratinization only in upper and/or lower jaw sheath), TR (tadpoles with lack of keratinization, with non-disrupted supporting tissue, only in upper and/or lower tooth rows), and JT (tadpoles with lack of keratinization in jaw sheaths and tooth rows) (Fig 1). All tadpole specimens were deposited in the Amphibia—Tadpoles collection of the Department of Zoology and Botany of UNESP–São José do Rio Preto (DZSJRP-Amphibia-Tadpoles).
Fig 1. Example of oral deformities found among the studied tadpoles.
(A) and (B) = normal tadpoles; (C) = tadpole with dekeratinized jaw sheaths (black arrows) and generalized dekeratinization in tooth rows; (D) = tadpole with dekeratinized upper jaw sheath (black arrow). Upper images Boana albopunctata, and lower images Scinax hayii.
Batrachochytrium dendrobatidis detection
After the oral discs of all tadpoles were inspected, a subsample, composed of all the individuals that possessed oral deformities (N = 195) and 184 randomly selected individuals without oral deformities, was analyzed for Bd detection. Oral discs of selected tadpoles were excised and air-dried on filter paper as described in Hyatt et al. [39]. DNA oral disc extraction was performed using PrepMan Ultra (Applied Bioysistems) and amplified using a CFX96TM Real-Time PCR Detection System (Bio-Rad) with a Bd-specific Taqman Assay [40]. Each 96-well assay plate included a negative control and four different standards containing DNA from 100, 10, 1 and 0.1 Bd genome equivalents. For all samples, the negative control and standards were run in duplicate. Samples that showed signs of inhibition (nonsigmoidal amplification) were further diluted to 1:100 and re-analyzed. Only the presence or absence of Bd was determined, and samples were considered Bd positive when both of the two duplicate analyses revealed Bd zoospore genome equivalents >0.1, and the amplification curves have a sigmoidal shape. If not, the sample was re-run and considered positive only with another positive result recorded. DNA analyses were carried out at Museo Nacional de Ciencias Naturales, CSIC (Madrid, Spain).
Statistical analyses
Two-tailed Fisher’s exact test was used to assess the association between individuals with oral deformities and infection with Bd for the total number of tadpoles analyzed for Bd presence of all six species sampled. We constructed contingency tables (2 x 2) with two binary variables: 1 = oral deformities present, 0 = oral deformities not present; and 1 = Bd infection, and 0 = no Bd infection.
Results
The tadpoles studied belonged to six different species: Aplastodiscus albosignatus (N = 721), Boana albopunctata (N = 793), Boana faber (N = 467), Scinax hayii (N = 80), Crossodactylus caramaschii (N = 34) and Physalaemus cuvieri (N = 61). At least one type of oral deformity was found in 9% (195) of the 2156 tadpoles inspected. Of the tadpoles that exhibited oral deformities, 51.3% had JT, while 29.2% had only TR, and 19.5% only JS.
There was no relationship between Bd infection and tooth rows dekeratinization (TR) in any of studied of species (Table 1). However, dekeratinization of the jaw sheath, accompanied or not with dekeratinization of teeth (JS or JT), was related to Bd infection in three of the species studied: A. albosignatus showed a nonrandom relationship with JS (Fisher’s exact test; N = 197, two-tailed p = 0.0023) and JT (N = 197, two-tailed p < 0.001); B. albopunctata showed nonrandom relationship with JT (N = 63, two-tailed p < 0.001); and S. hayii showed nonrandom relationships with JS (N = 32, two-tailed p = 0.0068). By contrast, B. faber, C. caramaschii and P. cuvieri did not exhibit relationship between any of the oral deformities studied and Bd infection (Table 1).
Table 1. Oral deformities and related Batrachochytrium dendrobatidis (Bd) infections of tadpoles of six species of Atlantic Forest anurans in southeastern Brazil.
| JS | TR | JT | JSD-Proxy | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ns | Na | NBd+ | Nd | NBd+ | P-value | Nd | NBd+ | P-value | Nd | NBd+ | P-value | Nd | NBd+ | P-value | |
| Aplastodiscus albosignatus | 721 | 197 | 124 | 27 | 24 | 0.0023 | 25 | 6 | < 0.001a | 78 | 73 | < 0.001 | 105 | 97 | < 0.001 |
| Boana albopunctata | 793 | 63 | 12 | 1 | 1 | 0.1904 | 20 | 0 | 0.0124a | 12 | 8 | < 0.001 | 13 | 9 | < 0.001 |
| Boana faber | 467 | 33 | 8 | 2 | 2 | 0.0530 | 5 | 1 | 0.9999 | 0 | 0 | 0.9999 | 2 | 2 | 0.0530 |
| Scinax hayii | 80 | 32 | 16 | 7 | 7 | 0.0068 | 2 | 1 | 0.9999 | 4 | 4 | 0.1012 | 11 | 11 | < 0.001 |
| Crossodactylus caramaschii | 34 | 34 | 5 | 1 | 0 | 0.9999 | 2 | 0 | 0.9999 | 5 | 2 | 0.1464 | 6 | 2 | 0.2053 |
| Physalaemus cuvieri | 61 | 20 | 1 | 0 | 0 | 0.9999 | 3 | 0 | 0.9999 | 1 | 0 | 0.9999 | 1 | 0 | 0.9999 |
| TOTAL | 2156 | 379 | 158 | 38 | 34 | < 0.001 | 57 | 8 | < 0.001a | 100 | 87 | < 0.001 | 138 | 121 | < 0.001 |
Significant p-values of two tailed Fisher’s exact tests (in bold) indicate a non-random relationship between presence of oral deformities and Bd infection. Ns = tadpoles studied; Na = tadpoles analyzed for Bd detection; NBd+ = tadpoles infected by Bd; Nd = tadpoles with oral deformities corresponding to JS = lack of keratinization only in jaw sheaths, TR = lack of keratinization, with non-disrupted supporting tissue, only in tooth rows, JT = lack of keratinization in jaw sheaths and tooth rows, and JSD-Proxy = jaw sheath dekeratinization with or without dekeratinization in tooth rows.
a Significant relationship but in the opposite direction (absence of TR deformity related to presence of Bd).
In the light of these results, we decided to sum JS and JT into a single proxy, JSD-proxy (JSD = jaw sheaths dekeratinization with or without dekeratinization in tooth rows), in order to simplify the determination of oral dekeratinization of tadpoles in future studies. Same statistical analyses performed for TR, JS and JT were then done for this JSD-proxy. The results showed that JSD-proxy considerably improved the accuracy of identifying Bd infection. Our original proxies (JS, TR and JT) included 33.8% false positives and 20.1% false negatives for Bd infection; the JSD-proxy, on the other hand, included only 12.3% false positives and 18.7% false negatives (see details in Table 2). False positives corresponded to tadpoles that possessed oral deformities but were negative for Bd infection via qPCR analysis. False negatives, on the other hand, corresponded to tadpoles that possessed normal oral mouthparts but were positive for Bd infection via qPCR.
Table 2. Cases of false positives and false negatives for the detection of Batrachochytrium dendrobatidis (Bd) infection produced by the use of the three oral deformities studied as proxies (JS, TR and JT) and for the JSD-proxy.
| JS, TR and JT | JSD-Proxy | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nd | False positives | Nn | False negatives | Nd | False positives | Nn | False negatives | |||||
| n | % | n | % | n | % | n | % | |||||
| Aplastodiscus albosignatus | 130 | 27 | 20.8 | 67 | 21 | 31.3 | 105 | 8 | 7.6 | 92 | 27 | 29.3 |
| Boana albopunctata | 33 | 24 | 72.7 | 30 | 3 | 10.0 | 13 | 4 | 30.8 | 50 | 3 | 6.0 |
| Boana faber | 7 | 4 | 57.1 | 26 | 5 | 19.2 | 2 | 0 | 0.0 | 31 | 6 | 19.3 |
| Scinax hayii | 13 | 1 | 7.7 | 19 | 4 | 21.1 | 11 | 0 | 0.0 | 21 | 5 | 23.8 |
| Crossodactylus caramaschii | 8 | 6 | 75.0 | 26 | 3 | 11.5 | 6 | 4 | 66.7 | 28 | 3 | 10.7 |
| Physalaemus cuvieri | 4 | 4 | 100 | 16 | 1 | 6.25 | 1 | 1 | 100 | 19 | 1 | 5.26 |
| TOTAL | 195 | 66 | 33.8 | 184 | 37 | 20.1 | 138 | 17 | 12.3 | 241 | 45 | 18.7 |
Nd = tadpoles with oral deformities corresponding to JS = lack of keratinization only in jaw sheaths, TR = lack of keratinization, with non-disrupted supporting tissue, only in tooth rows, JT = lack of keratinization in jaw sheaths and tooth rows, and JSD-Proxy = jaw sheath dekeratinization with or without dekeratinization in tooth rows; Nn = normal tadpoles.
Although the use of JSD-Proxy was more accurate at identifying Bd infection in tadpoles than our original proxies, the analyses of the relationships between Bd infection and JSD-proxy found a nonrandom relationship only for three species of the family Hylidae: A. albosignatus (Fisher’s exact test; N = 197, two-tailed p < 0.001), B. albopunctata (N = 63, two-tailed p<0.001), and S. hayii (N = 32, two-tailed p < 0.001) (see details in Table 1).
Discussion
This is the first study to report the presence of Bd in Núcleo Curucutu, one of the largest and most preserved remnants of Atlantic Forest in São Paulo State, which is also a refuge to one of the most biodiverse amphibian faunas of a single locality [41]. Therefore, this virtually pristine area is added to the many others around the world that possess Bd (e.g. [18, 42]).
Not all of the tadpoles that possessed oral deformities were infected by Bd, and so some of the factors that caused the deformities in our studied tadpoles remain unknown and deserve further investigation. Since our study area is a very well preserved remnant of Atlantic Forest, we considered that one might rule out chemical contamination as the cause of deformities. More importantly, our results showed that TR was the worst proxy for Bd infection; in fact, of the 57 tadpoles exhibited only TR deformities, only 8 (14%) were infected with Bd. On the other hand, of the 138 tadpoles possessing dekeratinization of the jaw sheaths, accompanied or not with dekeratinization of the teeth, 121 (87.7%) were infected. These results are similar to those obtained by Knapp and Morgan [26] and Vieira et al. [28], who also found a strong correlation between jaw sheaths dekeratinization and Bd infection. This finding is also consistent with Marantelli et al. [24], who found that the dekeratinization of jaw sheaths indicates a heavier infection than dekeratinization of tooth rows.
Despite the fact that the analyses of all studied tadpoles together found a strong relationship between tadpoles possessing dekeratinization of jaw sheaths and Bd infection, the results by species were less clear. Only three species of the family Hylidae exhibited a significant relationship between JSD-Proxy and Bd infection, which was not observed for B. faber (Hylidae), C. caramaschii (Hylodidae) and P. cuvieri (Leptodactylidae). These results agree with Blaustein et al. [31], who also detected varying relationships between Bd infection and oral deformities among species. Therefore, and in discordance with Padget-Flohr and Goble [30], who concluded that Bd infection and anuran larval mouthpart deformities are two separate processes, we found that a relationship between dekeratinization in jaw sheaths and Bd infection does indeed exist in three species of family Hylidae.
Although the relationship between jaw sheaths and Bd infection was found for some of our studied species, the use of JSD-proxy remains unreliable for purposes of assessing Bd prevalence in tadpoles populations. Based on this proxy, Bd detection in species that exhibited a positive relationship with jaw sheaths dekeratinization resulted in up to 30.8% false positives in B. albopunctata and up to 29.3% false negatives in A. albosignatus. Furthermore, the use of the JSD-proxy in species for which no relationship was found, reached 100% of false positives. Further illustrating the unreliability of JSD-proxy, in an open area pond where we sampled 23 tadpoles of B. albopunctata (two of them with dekeratinized jaw sheaths), the use of JSD-proxy would have classified the pond as a Bd positive site although qPCR analyses did not confirm this assumption.
Thus, we conclude that the use of oral dekeratinization as a generalized proxy for Bd detection in tadpoles should not be used as a single diagnosis technique. We then recommend the use of more accurate techniques such as histology, histochemistry, or PCR analyses in order to obtain accurate diagnosis. However, it is worth noting the usefulness of using JSD-proxy as a screening tool in those species for which the relationship between Bd-infection and oral dekeratinization has been proven, since its use minimize the number of individuals evaluated by more costly or time-intensive methods.
Acknowledgments
We are in debt to FS Annibale, LR Malagoli, CE Sousa and D Garcia for their help in fieldwork, K Picheli for her help with tadpole identification and C Monsalve for her help with qPCR analyses.
Data Availability
All relevant data are within the paper.
Funding Statement
ANL thanks Asociación Universitaria Iberoamericana de Postgrado for doctoral fellowship (www.auip.org); DSD thanks Coordenação de Aperfeiçoamento de Pessoal de Nível Superior for doctoral fellowship (#1518162) (www.capes.gov.br); DCRF thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico for the research fellowship (303522/2013-5) and (563075/2010-4) (www.cnpq.br), and Fundação de Amparo à Pesquisa do Estado de São Paulo (#2010/52321-7) (www.fapesp.br); JB thanks Fundación BBVA (www.fbbva.es); RJS thanks Fundação de Amparo à Pesquisa do Estado de São Paulo (#2014/23677-9), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (304929/2015-8). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Baillie JEM, Griffiths J, Turvey ST, Loh J, Collen B. Evolutions Lost: Status and Trends of the World’s Vertebrates. 1st ed. Zoological Society of London, United Kingdom; 2010. [Google Scholar]
- 2.IUCN 2016. The IUCN Red List of Threatened Species. Version 2016–3. Table 3a: Status category summary by major taxonomic group (animals). Downloaded on 7 March 2017. Available from: http://www.iucnredlist.org/about/summary-statistics#Tables_3_4
- 3.Bradford DF, Tabatabai F, Graber DM. Isolation of remaining populations of the native frog, Rana muscosa, by introduced fishes in Sequoia and Kings Canyon National Parks, California. Conserv Biol. 1993;7: 882–888. [Google Scholar]
- 4.Fisher RN, Shaffer HB. The decline of amphibians in California's Great Central Valley. Conserv Biol. 1996:10: 1387–1397. [Google Scholar]
- 5.Bradford D. Allotopic distribution of native frogs and introduced fishes in high Sierra Nevada lakes of California: implication of the negative effect of fish introductions. Copeia. 1989;3 775–778. [Google Scholar]
- 6.Harte J, Hoffman E. Possible effects of acidic deposition on a Rocky Mountain population of the tiger salamander Ambystoma Tigrinum: Conserv Biol. 1989;3: 149–158 [Google Scholar]
- 7.Blaustein AR, Hoffman PD, Hokit DG, Kiesecker JM, Walls SC, Hays JB. UV repair and resistance to solar UV-B in amphibian eggs: A link to population declines? Proc Natl Acad Sci USA. 1994;91: 1791–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaustein AR, Kiesecker JM, Chivers DP, Anthony RG. Ambient UV-B radiation causes deformities in amphibian embryos. Proc Natl Acad Sci USA. 1997;94: 13735–13737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lizana M, Pedraza EM. The effects of UV-B radiation on toad mortality in mountainous areas of central Spain. Conserv Biol. 1998;12: 703–707. [Google Scholar]
- 10.Heyer RW, Rand SA, Cruz CAG, Peixoto OL. Decimations, extinctions, and colonizations of frog populations in southeast Brazil and their evolutionary implications. Biotropica. 1988;20, 230–235. [Google Scholar]
- 11.Laurence WF. Catastrophic declines of Australian rainforest frogs: Is unusual weather responsible? Biol Conserv. 1996; 77: 203–212. [Google Scholar]
- 12.Pounds JA, Fogden MPL, Campbell JH. Biological response to climate change on a tropical mountain. Nature. 1999;398: 611–615 [Google Scholar]
- 13.Carey C. Hypothesis concerning the causes of the disappearance of boreal toads from the mountains of Colorado. Conserv Biol. 1993;7: 355–362. [Google Scholar]
- 14.Kiesecker JM, Blaustein AR. Synergism between UV-B radiation and a pathogen magnifies amphibian embryo mortality in nature. Proc Natl Acad Sci USA. 1995;92: 11049–11052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger L, Speare R, Daszak P, Green DE, Cunningham AA, Goggin CL, et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci USA. 1998;95: 9031–9036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daszak P, Berger L, Cunningham AA, Hyatt AD, Green DE, Speare R. Emerging infectious disease and amphibian population declines. Emerg Infect Dis. 1999;5: 735–748. doi: 10.3201/eid0506.990601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosa GM, Sabino-Pinto J, Laurentino TG, Martel A, Pasmans F, Rebelo R, et al. Impact of asynchronous emergence of two lethal pathogens on amphibian assemblages. Sci Rep. 2017; 7, 43260; doi: 10.1038/srep43260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosch J, Martinez-Solano I, Garcia-Paris M. Evidence of a chytrid fungus infection involved in the decline of the common midwife toad (Alytes obstetricans) in protected areas of central Spain. Biol Conserv. 2001;97: 331–7. [Google Scholar]
- 19.Lips KR, Brem F, Brenes R, Reeve JD, Alford RA, Voyles J. Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc Natl Acad Sci USA. 2006;103(9): 3165–3170. doi: 10.1073/pnas.0506889103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skerratt LF, Berger L, Speare R, Cashins S, McDonald KR, Phillott AD, et al. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth. 2007. doi: 10.1007/s10393-007-0139-8 [Google Scholar]
- 21.McDiarmid RW, Altig R. Tadpoles: the biology of anuran larvae 1st ed. The University of Chicago Press; 1999. [Google Scholar]
- 22.Berger L, Speare R, Hyatt A. Chytrid fungi and amphibian declines: Overview, implications and future directions In: Declines and disappearances of Australian frogs. Published by Environment Australia; 1999. [Google Scholar]
- 23.Nichols DK, Lamirande EW, Pessier AP, Longcore JE. Experimental transmission of cutaneous chytridiomycosis in dendrobatid frogs. J Wildl Dis. 2001;37(1): 1–11. doi: 10.7589/0090-3558-37.1.1 [DOI] [PubMed] [Google Scholar]
- 24.Marantelli G, Berger L, Speare R, Keegan L. Distribution of the amphibian chytrid Batrachochytrium dendrobatidis and keratin during tadpole development. Pac Conserv Biol. 2004;10: 173–79. [Google Scholar]
- 25.Fellers GM, Green DE, Longcore JE. Oral chytridiomycosis in the mountain yellow-legged frog (Rana muscosa). Copeia. 2001: 945–953. [Google Scholar]
- 26.Knapp RA, Morgan JAT. Tadpole mouthpart depigmentation as an accurate indicator of chytridiomycosis, an emerging disease of amphibians. Copeia. 2006;2: 188–197. [Google Scholar]
- 27.Drake DL, Altig R, Grace JB, Walls SC. Occurrence of oral deformities in larval anurans. Copeia. 2007;2: 449–458. [Google Scholar]
- 28.Vieira CA, Toledo LF, Longcore JE, Longcore JR. Body length of Hylodes cf. ornatus and Lithobates catesbeianus tadpoles, depigmentation of mouthparts, and presence of Batrachochytrium dendrobatidis are related. Braz J Biol. 2013;73(1): 195–199. [DOI] [PubMed] [Google Scholar]
- 29.Carvalho T, Becker CG, Toledo LF. Historical amphibian declines and extinctions in Brazil linked to chytridiomycosis. Proc R Soc B. 2017. 284:20162254 http://dx.doi.org/10.1098/rspb.2016.2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Padgett-Flohr GE, Goble ME. Evaluation of tadpole mouthpart depigmentation as a diagnostic test for infection by Batrachochytrium dendrobatidis for four California anurans. J Wildl Dis. 2007;43(4): 690–699. doi: 10.7589/0090-3558-43.4.690 [DOI] [PubMed] [Google Scholar]
- 31.Blaustein AR, Romansic JM, Scheessele EA, Han BA, Pessier AP, Longcore JE. Interspecific variation in susceptibility of frog tadpoles to the pathogenic fungus Batrachochytrium dendrobatidis. Conserv Biol. 2005;19(5): 1460–1468. doi: 10.1111/j.1523-1739.2005.00195.x [Google Scholar]
- 32.Rachowicz LJ. Mouthpart pigmentation in Rana muscosa tadpoles: seasonal changes without chytridiomycosis. Herpetol Rev. 2002;33: 263–265. [Google Scholar]
- 33.Rowe CL, Kinney OM, Congdon JD. Oral deformities in tadpoles of the bullfrog (Rana catesbeiana) caused by conditions in a polluted habitat. Copeia. 1998;1: 244–246. [Google Scholar]
- 34.Brett MAS, Gouchie GM, Wassersug RJ. Can visual stimulation alone induce phenotypically plastic responses in Rana sylvatica tadpole oral structures? J Herpetol. 2009;43(1): 165–168. [Google Scholar]
- 35.Relyea RA, Auld JR. Predator and competitor induced plasticity: how changes in foraging morphology affect phenotypic trade-offs. Ecology. 2005;86(7): 1723–1729. [Google Scholar]
- 36.Garcia RJF, Pirani JR. Análise sobre a interferência antrópica na origem dos campos do Núcleo Curucutu, Parque Estadual da Serra do Mar, São Paulo. Paisagem Ambiente: ensaios. 2005; 20: 131–151. [Google Scholar]
- 37.Altig R. Comments on the descriptions and evaluations of tadpole mouthpart anomalies. Herpetol Conserv Biol. 2007;2(1):1–4. [Google Scholar]
- 38.Gosner K. L. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica. 1960;16(3):183 [Google Scholar]
- 39.Hyatt AD, Boyle DG, Olsen V, Boyle DB, Berger L, Obendorf D, et al. Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Dis Aquat Org. 2007;73: 175–192. doi: 10.3354/dao073175 [DOI] [PubMed] [Google Scholar]
- 40.Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Org. 2004;60: 141–148. doi: 10.3354/dao060141 [DOI] [PubMed] [Google Scholar]
- 41.Malagoli LR. Diversidade e distribuição dos anfíbios anuros do Núcleo Curucutu, Parque Estadual da Serra do Mar, SP. M. Sc. Thesis, UNESP. 2013.
- 42.Lips KR. Mass mortality and population declines of anurans at an upland site in western Panama. Conserv Biol. 1999;13(1): 117–125. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.