Figure 1.
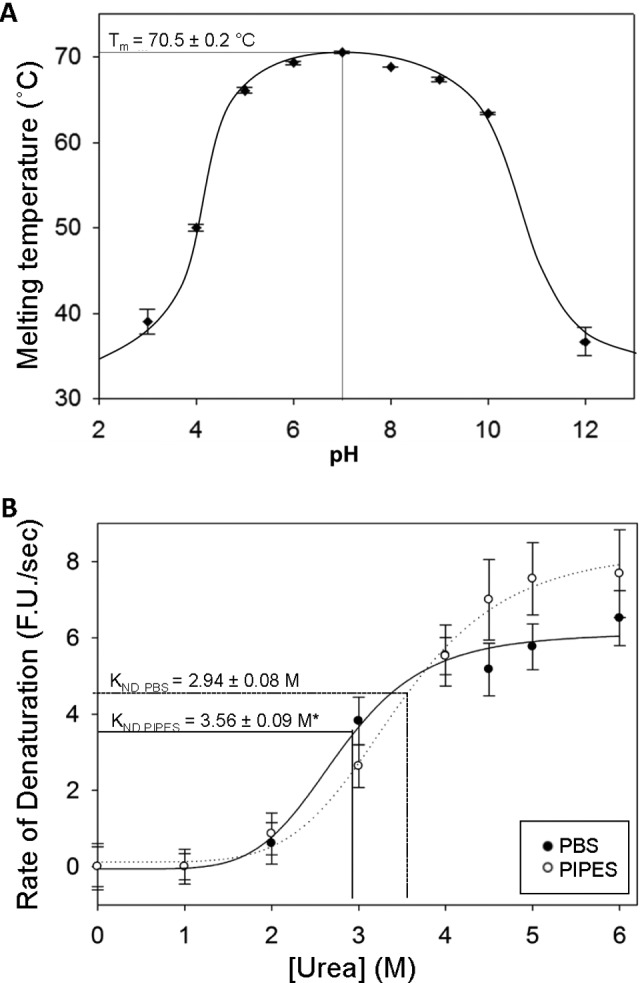
The effect of buffer species and environmental pH on immunoglobulin G (IgG) stability against denaturation by heat and urea. A, Unfolding temperatures (T m) of human IgG over a range of pH conditions in various buffers (see Table 1 for list) as determined by differential scanning fluorimetry (DSF) with a heating rate of 0.5°C/30 s. Optimal thermal stability of IgG was observed at pH 7.0, with a stable range of pH 5.0 to 10.0. B, Effect of buffer species on the velocity of denaturation of IgG as determined by the rate of SYPRO Orange fluorescence increases at various concentrations of urea. Immunoglobulin G samples were incubated at 4°C in either phosphate-buffered saline (PBS) or 1,4-Piperazinediethanesulfonic acid (PIPES) buffer (both pH 7.0), with various concentrations of urea. An asterisk (*) indicates significantly different from the PBS value (P < .01). Data for plots (A) and (B) represent mean ± standard error of mean (SEM; n = 4 trials). For both experiments, fluorescence (λex 485 nm; λem 625 nm) was measured using a modified BioRad iCycler thermocycler.
