Abstract
Log file–based methods are attracting increasing interest owing to their ability to validate volumetric-modulated arc therapy outputs with high resolution in the leaf and gantry positions and in delivered dose. Cross-validation of these methods for comparison with measurement-based methods using the ionization chamber/ArcCHECK-3DVH software (version 3.2.0) under the same conditions of treatment anatomy and plan enables an efficient evaluation of this method. In this study, with the purpose of cross-validation, we evaluate the accuracy of a log file–based method using Elekta log files and an X-ray voxel Monte Carlo dose calculation technique in the case of leaf misalignment during prostate volumetric-modulated arc therapy. In this study, 10 prostate volumetric-modulated arc therapy plans were used. Systematic multileaf collimator leaf positional errors (±0.4 and ±0.8 mm for each single bank) were deliberately introduced into the optimized plans. Then, the delivered 3-dimensional doses to a phantom with a certain patient anatomy were estimated by our system. These doses were compared with the ionization chamber dose and the ArcCHECK-3DVH dose. For the given phantom and patient anatomy, the estimated dose strongly coincided with the ionization chamber/ArcCHECK-3DVH dose (P < .01). In addition, good agreement between the estimated dose and the ionization chamber/ArcCHECK-3DVH dose was observed. The dose estimation accuracy of our system, which combines Elekta log files and X-ray voxel Monte Carlo dose calculation, was evaluated.
Keywords: patient-specific QA, log file, Monte Carlo, volumetric-modulated arc therapy, radiotherapy, oncology
Introduction
Volumetric-modulated arc therapy (VMAT) was developed to enable dynamic control of the multileaf collimator, jaw, and dose rate during gantry rotation. Thus, VMAT delivery technology allows for the generation of a highly modulated dose distribution over a shorter time frame than that observed in step-and-shoot or dynamic intensity-modulated radiotherapies. Using this delivery technique, VMAT output is evaluated to ensure that the patient effectively and safely receives radiotherapy through patient-specific quality assurance (QA) procedures.
For developing patient-specific QA procedures, a log file–based method is used to estimate irradiated VMAT output with a 3-dimensional (3D) dose using a combination of linac component statuses (ie, leaf and jaw positions and delivered doses) and dose calculations performed in treatment planning systems (TPS)1–4; examples of such systems include the Eclipse TPS (Varian Medical Systems, Palo Alto, California) with a Varian log file1,3 and the Pinnacle TPS (Philips Radiation Oncology Systems, Fitchburg, Wisconsin) with an Elekta log file. These methods can estimate the irradiated dose without a measurement device and allow additional machine availability.
However, one problem with log file–based methods is that the estimated dose is affected by the inaccuracy of the calculation implemented in the TPS for dose estimation. Thus, inaccurate dose calculations propagate discrepancies from the irradiated VMAT output to the estimated dose. Therefore, a log file–based method is desirable for estimating patient dose using a sufficiently accurate calculation. Previous data show that the X-ray voxel Monte Carlo (XVMC) dose calculation method implemented in a Monaco TPS (Elekta, Stockholm, Sweden) exhibits high accuracy5–9; moreover, the discrepancies between the calculated and measured doses in the XVMC method were found to be smaller than those in the kernel-based8 and Boltzmann equation solver–based dose calculations.9 Thus, XVMC dose calculation is suitable for log file–based methods.
The present report is among the first to implement a log file–based method employing an XVMC dose calculation in the Monaco TPS and to determine the accuracy of this method for leaf misalignment via cross-validation. The purpose of performing cross-validation is to compare our results with those obtained using a measurement-based method under the same conditions of treatment anatomy and plan, thereby allowing an efficient evaluation. In this study, the dose estimated using the log file–based method was cross-validated using ArcCHECK-3DVH (Sun Nuclear Corporation, Melbourne, Florida) doses, which are sufficiently accurate for clinical practice10–14 and ionization chamber doses.
Materials and Methods
Treatment Planning and Error Simulation
Optimized plans for 10 prostate VMAT treatments were generated by using Monaco TPS version 5.10 (Elekta, Stockholm, Sweden) with a dose of 74 Gy in 37 fractions and employing single-arc VMAT with a 10-MV photon beam of Synergy (Elekta). Error-induced plans were generated by modifying leaf positions systematically at every control point described in the optimized plans. Therefore, the different control points within the same plan have the same leaf error. There were 4 types of leaf errors with magnitudes of ±0.4 and ±0.8 mm in the isocentral plane for each single bank; they were added at each control point using in-house software. In this study, the same leaf errors were introduced into both the leaf banks. The leaf errors resulted in an expanded or restricted aperture depending on their respective error magnitudes. The total number of error-induced plan was 40 (4 error-induced plans for each optimized plan × 10 optimized plans).
Log File–Based Method Comprising an Elekta Log File and an XVMC Dose Calculation
Our log file–based method employs an Elekta log file and an XVMC dose calculation implemented in the Monaco TPS. X-ray voxel Monte Carlo calculations based on mass densities provide better accuracy than kernel-based and Boltzmann equation solver methods in homogeneous and heterogeneous regions.5–9 An Elekta log file contains the following machine dynamic parameters: leaf and gantry positions with precisions of 0.1 mm and 0.1° and a delivered dose with a precision of 0.1 monitor units. In this study, Elekta log files were recorded at 0.5-second intervals during VMAT irradiation at a continuously variable dose rate.
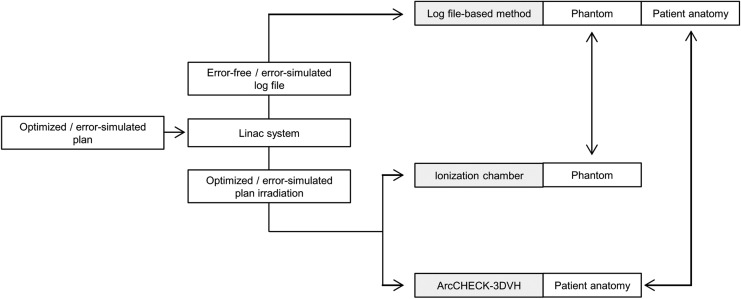
As shown in the upper part of Figure 1, to perform cross-validation of the log file–based dose using an ionization chamber/ArcCHECK-3DVH dose, an error-free log file was obtained during optimized plan irradiation. In addition, error-induced log files were obtained during error-induced plan irradiation. These log files were converted into the Monaco TPS original file format for each control point using an in-house program and subsequently imported to the Monaco TPS. For all log files, the mean doses to the CC13 ionization chamber (IBA Dosimetry, Schwarzenbruck, Germany) volume at its isocenter were estimated based on a computed tomography image set (18 cm × 18 cm × 18 cm) of a ImRT phantom (IBA dosimetry) that consists of water-equivalent material. The doses for each patient anatomy were also estimated. Dose estimation was performed using an XVMC dose calculation with a 2-mm dose grid and a 1.0% variance in the total beam.
Figure 1.
Flowchart of the cross-validation of the log file dose and ionization chamber/ArcCHECK-3DVH dose.
Ionization Chamber/ArcCHECK-3DVH Dose
For cross-validation of the ionization chamber doses (as shown in the middle part of Figure 1), optimized/error-induced plan doses to the chamber volume at the isocenter of the ImRT phantom were measured in a CC13 ionization chamber. Before measurement, the CC13 ionization chamber was cross-calibrated using a Farmer-type TN 30013 ionization chamber (PTW, Freiburg, Germany) with a 10 × 10 cm2 field.
For the ArcCHECK-3DVH dose, as shown in the lower part of Figure 1, first, the continuity of our ArcCHECK measurement with previous data was ensured. Two-dimensional (2D) gamma passing rates for optimized/error-induced plans were calculated for comparing the ArcCHECK measurements and the TPS-calculated dose. Subsequently, to obtain the irradiated 3D dose according to patient anatomy for each plan, these ArcCHECK measurements were exported to the 3DVH software (Sun Nuclear Corporation).
Cross-validation was performed on the phantom geometry by comparing the mean dose to the ionization chamber volume estimated using the log file–based method with that measured in the CC13 ionization chamber. In addition, for patient anatomy, the mean doses to the tumor and normal tissue volumes estimated using the log file-based method were compared with those obtained using ArcCHECK-3DVH and 3D gamma passing rate calculations.
Results
Basic ArcCHECK QA
For optimized/error-induced plans used in the 10 prostate cases, the mean 2D gamma passing rates for 3%/3 mm (3% absolute dose and 3 mm distance to agreement) and 2%/2 mm between the ArcCHECK measurement and the TPS dose are 99.1% ± 0.7% and 93.5% ± 2.5%, respectively. Note that 2D gamma calculations used the global percentage dose error normalization, and the low threshold dose for gamma analysis was set to 10% of the maximum dose. The measurement uncertainty function of the SNC software (Sun Nuclear Corporation) was not utilized.
Log File Dose Versus Ionization Chamber/ArcCHECK-3DVH Dose
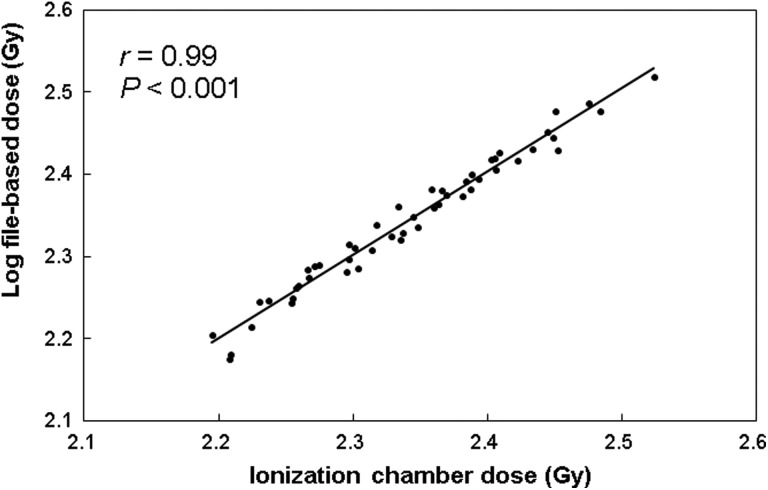
For the given phantom geometries, a cross-validation of the mean dose to the ionization chamber volume estimated using the log file–based method and that measured in the ionization chamber is shown in Figure 2. The correlation of the cross-validation result with Pearson correlation coefficient r was considered weak for r < 0.4, moderate for r = 0.4 to 0.7, and strong for r > 0.7. The r in this case was 0.99 (statistical significance is confirmed for Pearson r value: P < .001), indicating that the estimated chamber dose is in good agreement with measured chamber dose. For 10 patients, the mean difference between the estimated and measured dose values across all cases was 0.00% ± 0.01%. In this study, each dose–volume histogram (DVH) value was quantified using only the 3DVH application because Tyagi et al showed that quantified patient–organ dose values differ depending upon the application used.15
Figure 2.
For the phantom geometry, the mean dose to the ionization chamber volume estimated using the log file–based method versus the ionization chamber measurement.
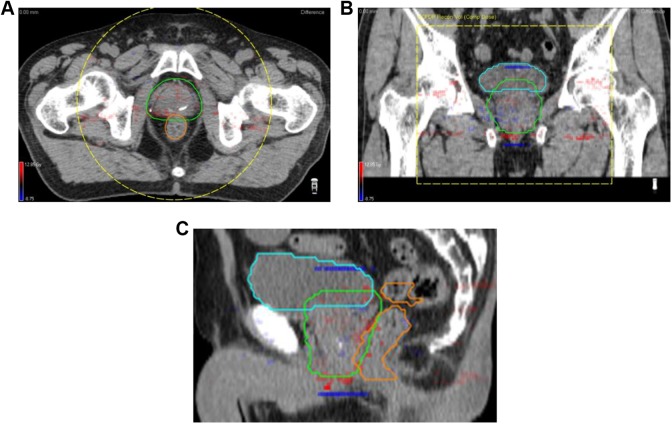
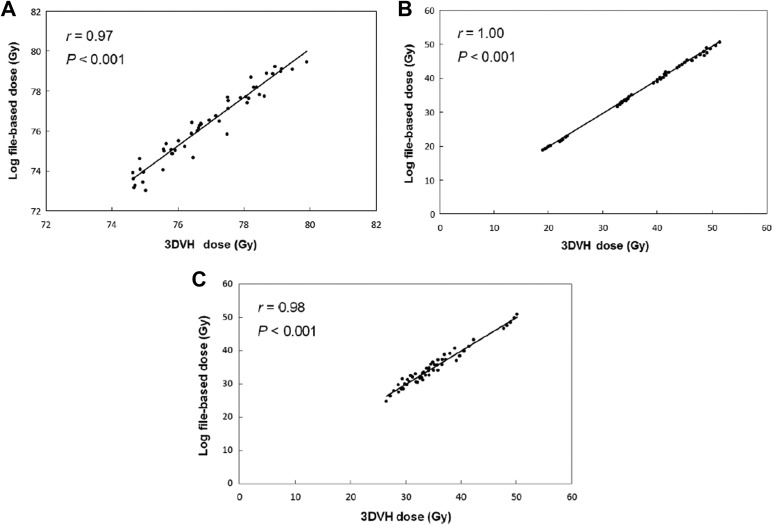
Similar to the results obtained for the phantom geometry, good agreement was found between the estimated and obtained patient doses under varying patient anatomy. Figure 3 shows a typical case of the 3D gamma distribution (1%/2 mm, global, and the low threshold was 20%). For the 10 prostate cases, the mean 3D gamma passing rate between the patient dose estimated using the log-file-based method and the obtained one was 96.0% ± 1.2%. The estimated dose also strongly coincided and showed a relatively good agreement with the ArcCHECK-3DVH dose. As shown in Figure 4, strong agreements between the mean doses to tumor and normal tissue volumes estimated using the log file–based method and ArcCHECK-3DVH were observed; r was 0.97 for the planning target volume (PTV), 1.00 for the bladder, and 0.98 for the rectum (P < .001). The mean difference ± 1 standard deviation (maximum difference) was 0.71 ± 0.7 Gy (2.62 Gy) for the PTV, 1.08 ± 1.0 Gy (3.51 Gy) for the bladder, and 0.21 ± 3.4 Gy (7.41 Gy) for the rectum.
Figure 3.
For the patient anatomy, the gamma index distributions (1%/2 mm, global, 20% dose threshold) between the log file and ArcCHECK-3DVH doses for typical cases; (A) axial plane, (B) coronal plane, and (C) sagittal plane. The planning target volume was contoured in green line, bladder was blue line, and rectum was orange line.
Figure 4.
For the phantom geometry, the mean dose to tumor/normal tissue volumes estimated using the log file–based method versus that obtained using ArcCHECK-3DVH; (A) planning target volume, (B) bladder, and (C) rectum.
Discussion
In this study, we established a log file–based patient–dose estimation system that uses an Elekta log file and an XVMC calculation implemented in the Monaco TPS. The major advantage of XVMC is that it enhances the accuracy of dose estimation. No significant differences between the doses estimated using our log file–based method and the ionization chamber/ArcCHECK-3DVH doses for both phantoms and patients were found, and these doses exhibited a strong correlation. The 3DVH software uses the 2D dose measured by the diodes implemented in ArcCHECK to obtain the 3D dose. The agreement between the 2D measured dose and the TPS dose for both optimized and error-induced plans in this study was consistent with the results of previous research.16,17 The agreement for prostate cases using the 2%/2 mm criterion was shown to be 99.7% by Nelms et al.16 Similarly, Kozelka et al demonstrated it to be 96.8%.17
In this study, systematic leaf error was applied as the error pattern because it was more suitable for determining the accuracy of our log file–based method instead of the random leaf bank error, in which each open leaf position at each control point is modified by a number generated from a truncated Gaussian distribution centered on zero using a user-defined standard deviation. As a result, the dosimetric change arising from the optimized dose to error-induced plan dose due to systematic leaf bank errors was significantly larger than that due to random leaf bank error. For example, with respect to clinical target volume, Rangel and Dunscombe18 reported 2.72% discrepancies due to systematic leaf bank errors and −0.01% discrepancies due to random leaf bank shift. Therefore, for clinical use, the data validation by systematic leaf shift pattern in this study was sufficient as it does not underestimate the values.
It is important to note that dose estimation errors were introduced into the estimated dose in the log file–based method even if dose estimation was performed using the XVMC dose calculation. Linac statuses recorded in log file–based methods are insensitive to miscalibration.19,20 For example, leaf positions can be detected using leaf position measurement sensors installed in the linac and recorded in a log file with calibration accuracy. Therefore, a leaf calibrated with a general tolerance level of ± 0.5 mm21 has leaf positional errors ≤0.5 mm. Previous studies have shown that the dose estimation error for a leaf with a position accuracy of ±0.5 mm for prostate VMAT is 1.22%, 0.95 Gy, and 0.45 Gy for the PTV, rectum, and bladder, respectively.19 However, in our data, the dose estimated using our log file–based method showed good agreement with the measured value. Because of this, in our clinic, the leaf was calibrated using an electronic portal imaging device–based fence test, which yields a miscalibration that is smaller than the general tolerance level.22,23 The more the leaf miscalibration is reduced, the more the reduction in the residual error.19 Therefore, good agreement of the measured dose with the ionization chamber/ArcCHECK-3DVH dose was obtained.
There are limitations to this study. The accuracy of our log file–based method was evaluated for a prostate VMAT with a comparatively simple plan optimization and delivery site. In more complex plans such as in the head and neck VMAT, highly modulated treatment beams can be generated using complex leaf and gantry motions. Therefore, even if XVMC dose calculation was applied in our log file–based method, spatial resolution of leaf and gantry positions recorded on log file or the recording interval time could have affected the accuracy of our log file–based method in more complex plans such as the head and neck VMAT.
Conclusion
In this study, we developed a log file–based method that uses an Elekta log file and an XVMC dose calculation implemented in the Monaco TPS. Our data show that the accuracies of the log file–based dose and ionization chamber/ArcCHECK-3DVH dose for leaf misalignment in the cases of both phantoms and patients were in good agreement with each other.
Abbreviations
- 2D
two dimensional
- 3D
three dimensional
- DVH
dose–volume histogram
- PTV
planning target volume
- QA
quality assurance
- TPS
treatment planning system
- VMAT
volumetric-modulated arc therapy
- XVMC
X-ray voxel Monte Carlo.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Foundation for Promotion of Cancer Research in Japan.
References
- 1. Calvo-Ortega JF, Teke T, Moragues S, Pozo M, Casals-Farran J. A Varian DynaLog file-based procedure for patient dose-volume histogram-based IMRT QA. J Appl Clin Med Phys. 2014;15(2):4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tyagi N, Yang K, Gersten D, Yan D. A real time dose monitoring and dose reconstruction tool for patient specific VMAT QA and delivery. Med Phys. 2012;39:7194–7204. [DOI] [PubMed] [Google Scholar]
- 3. Wijesooriya K, Aliotta E, Benedict S, Read P, Rich T, Larner J. RapidArc patient specific mechanical delivery accuracy under extreme mechanical limits using linac log files. Med Phys. 2012;39(4):1846–1853. [DOI] [PubMed] [Google Scholar]
- 4. Haga A, Nakagawa K, Shiraishi K, et al. Quality assurance of volumetric modulated arc therapy using Elekta Synergy. Acta Oncol. 2009;48(8):1193–1197. [DOI] [PubMed] [Google Scholar]
- 5. Carrasco P, Jornet N, Duch MA, et al. Comparison of dose calculation algorithms in phantoms with lung equivalent heterogeneities under conditions of lateral electronic disequilibrium. Med Phys. 2004;31(10):2899–2911. [DOI] [PubMed] [Google Scholar]
- 6. Krieger T, Sauer OA. Monte Carlo- versus pencil-beam-/collapsed-cone-dose calculation in a heterogeneous multi-layer phantom. Phys Med Biol. 2005;50(5):859–868. [DOI] [PubMed] [Google Scholar]
- 7. Arnfield MR, Siantar CH, Siebers J, Garmon P, Cox L, Mohan R. The impact of electron transport on the accuracy of computed dose. Med Phys. 2000;27(6):1266–1274. [DOI] [PubMed] [Google Scholar]
- 8. Fotina I, Winkler P, Kunzler T, Reiterer J, Simmat I, Georg D. Advanced kernel methods vs. Monte Carlo-based dose calculation for high energy photon beams. Radiother Oncol. 2009;93(3):645–653. [DOI] [PubMed] [Google Scholar]
- 9. Tan YI, Metwaly M, Glegg M, Baggarley S, Elliott A. Evaluation of six TPS algorithms in computing entrance and exit doses. J Appl Clin Med Phys. 2014;15(3):229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kadoya N, Saito M, Ogasawara M, et al. Evaluation of patient DVH-based QA metrics for prostate VMAT: correlation between accuracy of estimated 3D patient dose and magnitude of MLC misalignment. J Appl Clin Med Phys. 2015;16(3):5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coleman L, Skourou C. Sensitivity of volumetric modulated arc therapy patient specific QA results to multileaf collimator errors and correlation to dose volume histogram based metrics. Med Phys. 2013;40(11):111715. [DOI] [PubMed] [Google Scholar]
- 12. Vieillevigne L, Molinier J, Brun T, Ferrand R. Gamma index comparison of three VMAT QA systems and evaluation of their sensitivity to delivery errors. Phys Med. 2015;31(7):720–725. [DOI] [PubMed] [Google Scholar]
- 13. Opp D, Nelms BE, Zhang G, Stevens C, Feygelman V. Validation of measurement-guided 3D VMAT dose reconstruction on a heterogeneous anthropomorphic phantom. J Appl Clin Med Phys. 2013;14(4):4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Watanabe Y, Nakaguchi Y. 3D evaluation of 3DVH program using BANG3 polymer gel dosimeter. Med Phys. 2013;40(8):082101. [DOI] [PubMed] [Google Scholar]
- 15. Tyagi N, Yang K, Yan D. Comparing measurement-derived (3DVH) and machine log file-derived dose reconstruction methods for VMAT QA in patient geometries. J Appl Clin Med Phys. 2014;15(4):4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nelms BE, Opp D, Robinson J, et al. VMAT QA: measurement-guided 4D dose reconstruction on a patient. Med Phys. 2012;39(7 pt 1):4228–4238. [DOI] [PubMed] [Google Scholar]
- 17. Kozelka J, Robinson J, Nelms B, Zhang G, Savitskij D, Feygelman V. Optimizing the accuracy of a helical diode array dosimeter: a comprehensive calibration methodology coupled with a novel virtual inclinometer. Med Phys. 2011;38(9):5021–5032. [DOI] [PubMed] [Google Scholar]
- 18. Rangel A, Dunscombe P. Tolerances on MLC leaf position accuracy for IMRT delivery with a dynamic MLC. Med Phys. 2009;36(7):3304–3309. [DOI] [PubMed] [Google Scholar]
- 19. Katsuta Y, Kadoya N, Fujita Y, et al. Quantification of residual dose estimation error on log file-based patient dose calculation. Phys Med. 2016;32(5):701–705. [DOI] [PubMed] [Google Scholar]
- 20. Childress N, Chen Q, Rong Y. Parallel/Opposed: IMRT QA using treatment log files is superior to conventional measurement-based method. J Appl Clin Med Phys. 2015;16(1):5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Palta JR, Liu C, Li JG. Quality assurance of intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2008;71(suppl 1):S108–S112. [DOI] [PubMed] [Google Scholar]
- 22. Agnew A, Agnew CE, Grattan MWD, Hounsell AR, McGarry CK. Monitoring daily MLC positional errors using trajectory log files and EPID measurements for IMRT and VMAT deliveries. Phys Med Biol. 2014;59(9):N49–N63. [DOI] [PubMed] [Google Scholar]
- 23. Jorgensen MK, Hoffmann L, Petersen JB, Praestegaard LH, Hansen R, Muren LP. Tolerance levels of EPID-based quality control for volumetric modulated arc therapy. Med Phys. 2011;38(3):1425–1434. [DOI] [PubMed] [Google Scholar]