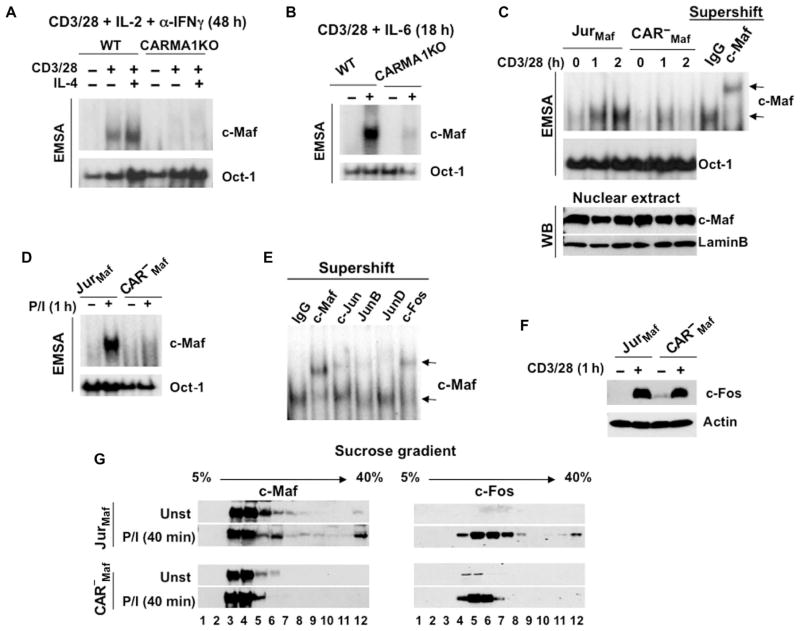
Fig. 2. CARMA1 is required for the activation of c-Maf.
(A and B) The DNA binding activity of c-Maf is defective in CARMA1-deficient CD4+ splenocytes. CD4+ T cells from the indicated mice were activated with plate-bound anti-CD3 and anti-CD28 antibodies in the presence of (A) IL-2 (30 U/ml), anti-IFNγ antibody (10 μg/ml), and recombinant murine IL-4 (10 ng/ml) for 48 hours or (B) IL-6 (20 ng/ml) for 18 hours. Nuclear extracts were prepared, and the binding activity of c-Maf was determined by EMSA. Data are representative of two experiments. (C and D) Jurkat cells (JurMaf) and CARMA1-deficient Jurkat cells (CAR−Maf) stably transfected with plasmid encoding c-Maf were stimulated with anti-CD3 and anti-CD28 antibodies or with P/I [PMA (20 ng/ml) and ionomycin (100 ng/ml)]. Nuclear extracts were prepared and analyzed by EMSA with 32P-labeled probes containing MARE motifs or Oct-1–binding sites. The specificity of c-Maf binding to the probe was confirmed by supershift with an anti–c-Maf antibody. Data are representative of three experiments. (E) Supershift of c-Maf dimers with different members of the AP-1 family. Jurkat cells stably expressing c-Maf were stimulated with soluble anti-CD3 and anti-CD28 antibodies. Nuclear extracts were incubated with the indicated antibodies for 30 min on ice and then were subjected to EMSA. (F) The abundance of c-Fos is intact in CARMA1-deficient cells. Jurkat cells and CARMA1-deficient Jurkat cells, both stably transfected with plasmid encoding c-Maf, were stimulated with anti-CD3 (3 μg/ml) and anti-CD28 (2 μg/ml) antibodies. Whole-cell lysates were subjected to Western blotting analysis with antibodies specific for c-Fos and actin. Data are representative of three experiments. (G) Fractionation of c-Maf–containing complexes. The indicated cells (20 × 106) were stimulated with P/I as described in (D), and whole-cell lysates were subjected to the sucrose density gradient centrifugation. Twelve fractions were collected and analyzed by Western blotting with antibodies specific for c-Maf or c-Fos. Blots are representative of two independent experiments.