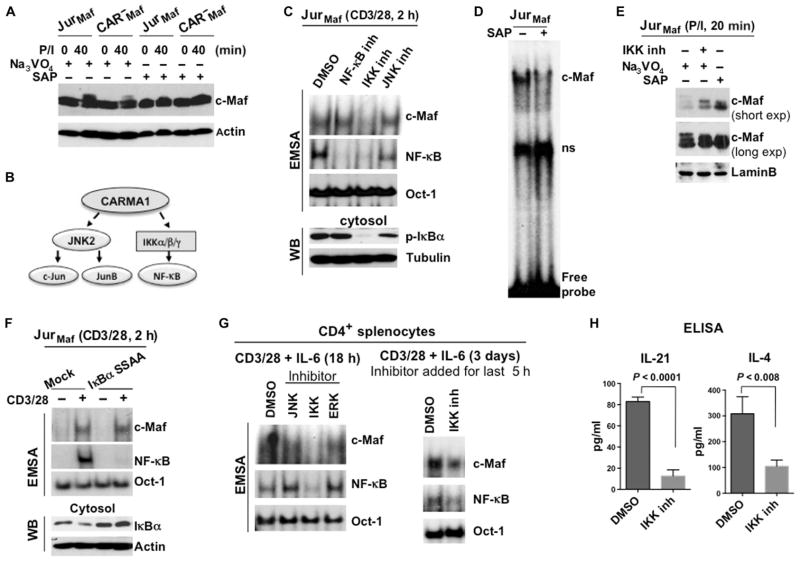
Fig. 3. The kinase activity of IKK is required for the activation of c-Maf.
(A) Analysis of the c-Maf phosphorylation status in Jurkat and CARMA1-deficient cells stably expressing c-Maf. The indicated cells were stimulated with P/I, and cell lysates were treated with Na3VO4 or SAP. The band shift in c-Maf was determined by Western blotting analysis. Blots are representative of two independent experiments. (B) Scheme outlining the requirement of CARMA1 for TCR-dependent IKK and JNK2 activity. (C) Effects of JNK and IKK inhibitors on the DNA binding activity of c-Maf. Jurkat cells stably expressing c-Maf were stimulated with antibodies against CD3 and CD28 in the presence of inhibitors of JNK (SP600125), IKK (TPCA-1), or the translocation of NF-κB for 2 hours. Nuclear extracts were then subjected to EMSA (upper panels). Nuclear and cytoplasmic fractions from the same cells were also analyzed by Western blotting (WB) with antibodies against the indicated proteins (middle and lower panels). Data are representative of three independent experiments. (D) Jurkat cells stably expressing c-Maf were stimulated with P/I, and nuclear extracts were treated with SAP before being subjected to gel-shift analysis. (E) Jurkat cells stably expressing c-Maf were stimulated with P/I in the presence or absence of IKK inhibitor. The indicated lysates were also treated with Na3VO4 or SAP. c-Maf band shifts were determined by Western blotting analysis, and both short and long exposures of the Western blots for c-Maf are shown. Data are representative of two independent experiments. (F) Jurkat cells stably expressing c-Maf were transfected with plasmid encoding the super repressor IκBα mutant (IκBα-S32A/S36A). Forty-eight hours later, the cells were stimulated with antibodies against CD3 and CD28 for 2 hours, and nuclear extracts were prepared and subjected to EMSA. The DNA binding activities of c-Maf and NF-κB were analyzed by EMSA with 32P-labeled probes containing c-Maf–, NF-κB–, or Oct-1–binding sites. Data are representative of two independent experiments. (G) CD4+ splenocytes from WT mice were stimulated by antibodies against CD3 and CD28 together with IL-6 in the presence of inhibitors of the indicated kinases for 18 hours (left) or for 3 days with an IKK inhibitor (right), which was added for the last 5 hours. The DNA binding activities of c-Maf and NF-κB were then analyzed by EMSA. Data are representative of two independent experiments. (H) CD4+ splenocytes from WT mice were stimulated with plate-bound antibodies against CD3 and CD28 for 48 hours. An IKK inhibitor or vehicle [dimethyl sulfoxide (DMSO)] was added for the last 36 hours of the stimulation (triplicate cultures for each condition), and media were collected. The amounts of IL-4 and IL-21 were measured by ELISA and are presented as means ± SD.