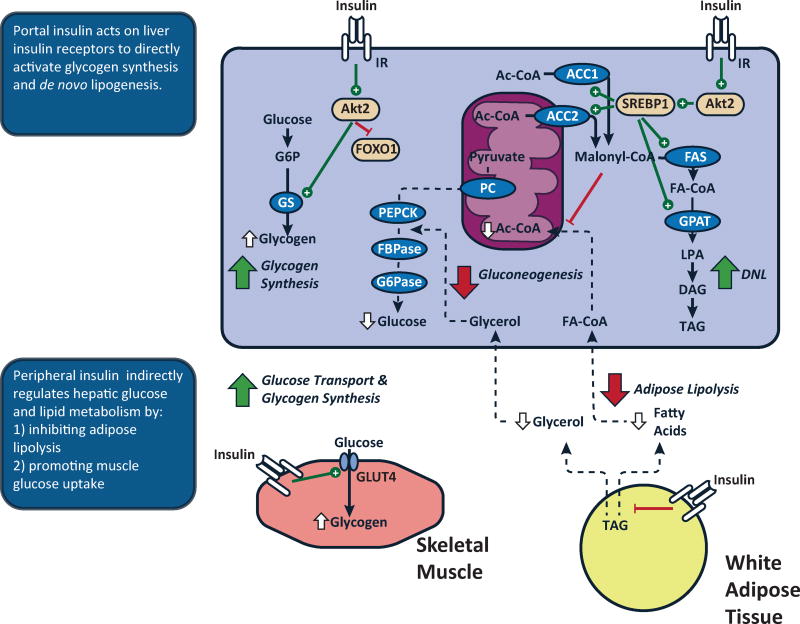
Figure 1. Insulin action regulates hepatic glucose and lipid metabolism via direct and indirect mechanisms.
The liver is exposed to a high concentration of insulin via the portal vein. Binding of insulin to the insulin receptor tyrosine kinase (IRTK) activates Akt2 and acutely activates glycogen synthase. Direct hepatic insulin action will also decrease transcription of gluconeogenic enzymes via inactivation of FOXO1, followed later by a decrease in the protein expression of these enzymes. Insulin signaling also promotes activation and expression of SREBP1. Peripheral insulin action also indirectly impacts hepatic glucose and lipid metabolism. In skeletal muscle, insulin activates glucose transport and glycogen synthesis, limiting glucose as a substrate for hepatic metabolism. In adipose tissue, insulin acts to promote glucose uptake and inhibit lipolysis. The latter decreases fatty acid and glycerol flux. The decrease in fatty acid flux decreases hepatic mitochondrial acetyl-CoA, an allosteric activator of pyruvate carboxylase. The decrease in glycerol flux will also decrease gluconeogenesis by constraining the influx of this substrate. The decrease in fatty acid flux will also decrease esterification of adipose-derived fatty acids.