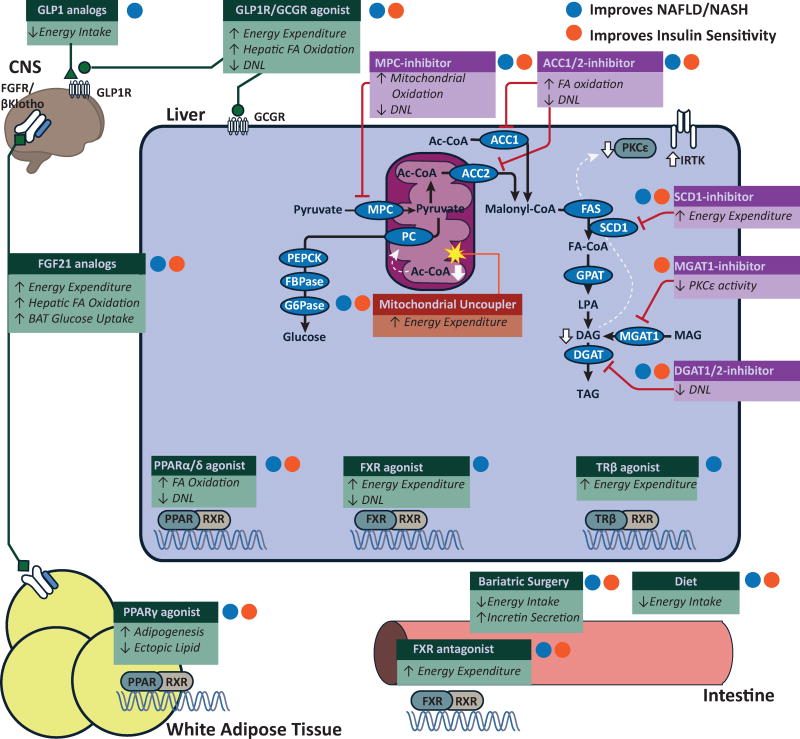
Figure 3. Potential targets to treat NAFLD and hepatic insulin resistance.
Putative targets to treat NAFLD and hepatic insulin resistance. Some therapeutic pathways (green boxes) broadly regulate metabolic pathways. Other therapeutic targets (purple boxes) are specific enzymes targets in lipid synthetic pathways. While many therapeutic targets indirectly increase energy expenditure, mitochondrial uncouplers (red boxes) directly increase cellular energy expditure decreasing cellular DAG and mitochondrial acetyl CoA which increase insulin signaling and decrease gluconeogenesis. White lines show pathways that have been reported decrease cellular DAG content and/or acetyl CoA concentration. Therapies that improve NAFLD/NASH are labeled with a blue dot. Therapies that improve insulin sensitivyt are labeled with an orange dot. Clinical trials have shown the efficacy of weight loss, bariatric surgery, GLP1 analogs (liraglutide) and PPARγ agonists (rosiglitazone, pioglitozatone). Current clinical investigation has explored the potential for PPARα/δ dual agonists (elafibranor), FXR agonists, FGF21 analogs (LY2405319, PF-052313023) and SCD1 inhibitors (Aramachol). Preclinical studies have shown possible efficacy of dual GLP1/GCGR agonists, intestinal FXR antagonists, inhibitors against MPC (MSDC-0602), ACC1/2 (ND-630), MGAT1 (MOGAT1 ASO), DGAT2 and glucagon-T3 hybrids (not depicted). Preclinical studies have also shown efficacy for liver-targeted mitochondrial uncoupling agents (CRMP) and other agents that increase cellular energy expenditure (e.g. salsalate, TTA, and niclosamide).