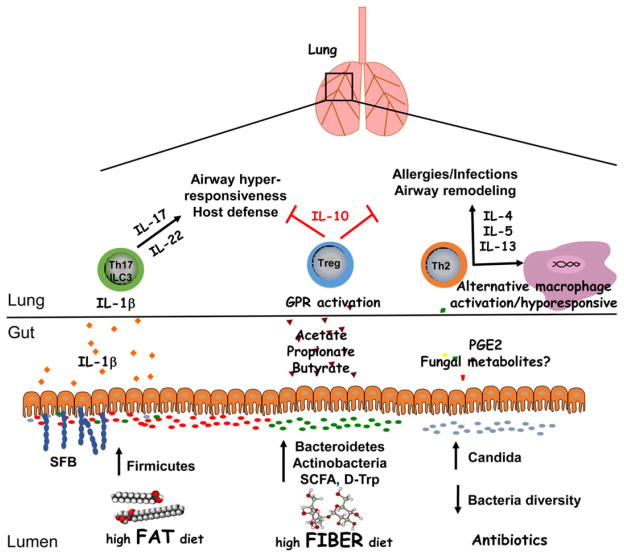
Figure 1.
Regulation of lung inflammation by intestinal microbiota. A diverse intestinal microbiota supports immune functions that are critical for maintaining homeostasis in the lungs. High fiber diets can increase the prevalence of Bacteroidetes and Actinobacteria species as well as the production of short chain fatty acids, which protect against airway inflammation through the induction of Tregs. Dysbiosis resulting from dietary fat or antibiotic use enhances lung inflammation in response to allergens or infections. Notably, an increased ratio of Firmicutes/Bacteroidetes species as well as segmented filamentous bacteria (SFB) colonization is associated with increased lung IL-17 and IL-22 responses. While these cytokines are important for host defense, they may contribute to AHR when directed against innocuous antigens. Antibiotic use can result in intestinal fungal overgrowth and increased blood concentrations of PGE2, leading to Th2 cell differentiation and alternative macrophage activation. Thus, antibiotics may inhibit the phagocytic capacity of alveolar macrophages, increasing susceptibility to opportunistic infections in the lungs, and promote Th2 responses to allergens. Overall, a lack of intestinal bacteria diversity may contribute to airway remodeling in patients with asthma. Future studies into the mechanistic relationship between diet, microbiota, genetics and lung inflammation may involve gnotobiotic models as well studying the effects of microbial metabolites on cell populations.