Abstract
Stereoselectivity in drug metabolism can not only influence the pharmacological activities, tolerability, safety, and bioavailability of drugs directly, but also cause different kinds of drug–drug interactions. Thus, assessing stereoselectivity in drug metabolism is of great significance for pharmaceutical research and development (R&D) and rational use in clinic. Although there are various methods available for assessing stereoselectivity in drug metabolism, many of them have shortcomings. The indirect method of chromatographic methods can only be applicable to specific samples with functional groups to be derivatized or form complex with a chiral selector, while the direct method achieved by chiral stationary phases (CSPs) is expensive. As a detector of chromatographic methods, mass spectrometry (MS) is highly sensitive and specific, whereas the matrix interference is still a challenge to overcome. In addition, the use of nuclear magnetic resonance (NMR) and immunoassay in chiral analysis are worth noting. This review presents several typical examples of drug stereoselective metabolism and provides a literature-based evaluation on current chiral analytical techniques to show the significance and challenges of stereoselectivity assessing methods in drug metabolism.
Keywords: Enantiomer, Chiral chromatography, Capillary electrophoresis, Mass spectrometry, NMR, Immunoassay
1. Introduction
In clinic, chiral drugs that contain at least one chiral center are widely used and play an important role in treating human diseases. Over half of therapeutic drugs are chiral, and the majority of them are administered as racemates, mixtures containing equal proportions of (R)- and (S)-enantiomers [1], [2]. Owing to the different three-dimensional configurations of enantiomers, although the individual drug enantiomers present identical physicochemical properties in an achiral environment, they generally show different pharmacological activities in a chiral environment, such as in the body [3]. The phenomenon that only one enantiomer is effective against a particular disease while the other enantiomer has different pharmacological activity or even toxicity exists commonly in many chiral drugs [4], [5]. For example, (R)-flurbiprofen can modulate γ-secretase and has the potential to treat the symptoms of Alzheimer's disease, while (S)-flurbiprofen is more toxic because it can inhibit cyclooxygenase directly [6], [7].
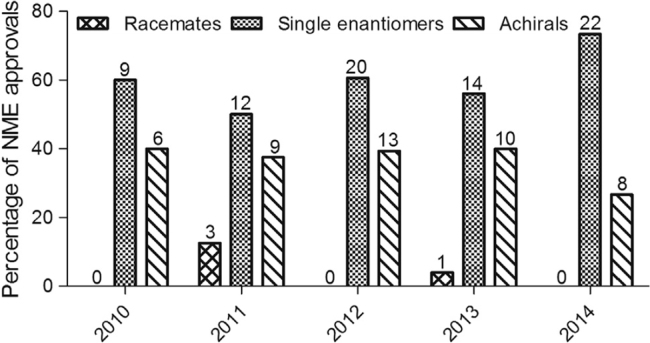
Thus, there has been an increased awareness of the effects of stereoselectivity in drug metabolism. Developing single enantiomer drugs has been a tendency in recent years due to their advantages, i.e., lower administered dose, simpler dose–response relationship and lower toxicity [3], [8]. Among the 127 new molecular entities (NMEs) approved by U.S. Food and Drug Administration (FDA) between January 2010 and December 2014, chiral NMEs were the major component (81 (64%) of the 127 NMEs), and among the 81 chiral NMEs, single enantiomers were the great majority (Fig. 1) [9].
Fig. 1.
The chirality of NMEs. The percentage (shown on the y-axis) and number (shown above the bars) of FDA-approved NMEs according to the chirality of the NME are shown for the 2010–2014 period.
In this case, many people doubt that the importance of stereoselectivity assessing in drug metabolism is limited and will steadily decline. However, it is essential to assess stereoselectivity in drug metabolism before we decide to develop a single-enantiomer or racemic drug. Nowadays, most countries' governments have stipulated that research on enantiomers should be carried out in pharmacology, toxicology and metabolism separately during the development of new drugs. Chiral drugs can be produced as racemates only if there is no obvious effect on the efficacy or toxicity when the two enantiomers coexist, because racemic drugs require lower costs of production but have more risks of application than single-enantiomer drugs. In addition, since many old drugs are still given as racemates, it is essential to monitor the blood concentration of each enantiomer respectively in therapeutic drug monitoring. Here, we review several typical examples of drug stereoselective metabolism from the aspects of fundamentals, types, and effects in order to further show that stereoselectivity assessing in drug metabolism is of great significance for pharmaceutical research and development (R&D) and the rational use in clinic. Additionally, current chiral analytical techniques, including high-performance liquid chromatography (HPLC), gas chromatography (GC), supercritical fluid chromatography (SFC), capillary electrophoresis (CE), nuclear magnetic resonance (NMR), and immunoassay, are evaluated. Although these techniques have made great contributions to stereoselectivity assessing, many challenges have not been overcome.
2. Stereoselectivity in drug metabolism
Among all pharmacokinetic processes, metabolism is the most stereoselective process due to the involvement of the enzymatic system, such as cytochrome P450 enzymes (CYPs) and uridine 5′-diphospho (UDP)-glucuronosyltransferases (UGTs). CYPs and UGTs are the major determinants during the metabolism of most drugs on the market [10], [11]. CYPs catalyze the oxidative reactions in Phase I metabolic reactions, while UGTs catalyze the glucuronidation reactions in Phase II metabolic reactions. They have a wide range of substrates and present great stereochemical sensitivity, i.e., different affinities and/or reactivities for two enantiomers of a chiral drug.
According to where the chiral discrimination in drug metabolism occurs, metabolic stereoselectivity can be classified into substrate stereoselectivity (the differential metabolism of two or more stereoisomeric substrates), product stereoselectivity (the differential formation of two or more stereoisomeric metabolites from a single substrate) and their combination, and substrate–product stereoselectivity, which contains a unique phenomenon, chiral inversion [12]. Some examples [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23] showing stereoselectivity in drug metabolism are presented in Table 1.
Table 1.
Stereoselectivity in drug metabolism.
| Drug | Main enzymatic metabolic pathway | Metabolic enzyme (Vmax(R)/(S)) | Ref. |
|---|---|---|---|
| Ifosfamide | 4-Hydroxylation | CYP2B1(0.59), CYP2B6(0.12), CYP3A4(2.55), CYP3A7(1.36) | [13] |
| N2-Dechloroethylation | CYP2B6(0.07), CYP3A4(6.69) | ||
| N3-Dechloroethylation | CYP2B6(2.41), CYP3A4(0.06) | ||
| N-Dechloroethylation | CYP2B1(1.60), CYP2B6(1.07), CYP3A4(0.21), CYP3A7(0.85) | ||
| Methadone | N-Dealkylation | CYP2B6(1.40), CYP3A4(2.75), CYP2C19(0.93) | [14] |
| Omeprazole | 5-Hydroxylation | CYP2C19(7.57) | [15], [16] |
| Sulfoxidation | CYP3A4(0.38) | ||
| 5′-O-Demethylation | CYP2C19(0.15) | ||
| Warfarin | 7-Hydroxylation | CYP2C9(<0.1), CYP2C19(>10) | [17] |
| 6-Hydroxylation | CYP1A2(>10) | ||
| 8-Hydroxylation | CYP2C9(<0.1), CYP2C19(>10) | ||
| 4′-Hydroxylation | CYP3A4(<0.1) | ||
| Verapamil | N-Demethylation | CYP2C8(0.46), CYP3A4(0.73), CYP3A5(1.20), CYP3A7(0.51) | [18], [19] |
| N-Dealkylation | CYP2C8(1.00), CYP3A4(1.27) | ||
| Metoprolol | O-Demethylation | CYP2D6(1.72) | [20] |
| Phenprocoumon | 4′-Hydroxylation | CYP2C9(3.30), CYP3A4(0.90) | [21] |
| 6-Hydroxylation | CYP2C9(0.65), CYP2C19(0.80), CYP3A4(0.64) | ||
| 7-Hydroxylation | CYP2C9(2.42), CYP2C19(0.52), CYP3A4(0.17) | ||
| Formoterol | Glucuronidation | UGT(0.61) | [22] |
| Salbutamol | Sulfation | M-PST(0.91) | [23] |
CYP: Cytochrome P450 enzyme, UGT: Uridine 5′-diphospho (UDP)-glucuronosyltransferase, PST: Phenolsulfotransferase.
2.1. Substrate stereoselectivity
Substrate stereoselectivity refers to the phenomenon that two enantiomers are metabolized at different rates in a reaction that neither creates nor adds a stereogenic element when forming the metabolites [12]. Enantiomers usually have different affinities with enzymes, which induces different metabolites and different metabolic rates. Therefore, they often show different pharmacological activities and elimination rates in the human body. In order to minimize toxicity and reduce the total dose of an administered drug, the majority of newly approved chiral drugs are not developed as racemates but as single enantiomers, which means that it is essential to study the substrate stereoselectivity of a chiral NME to decide which enantiomer should be produced.
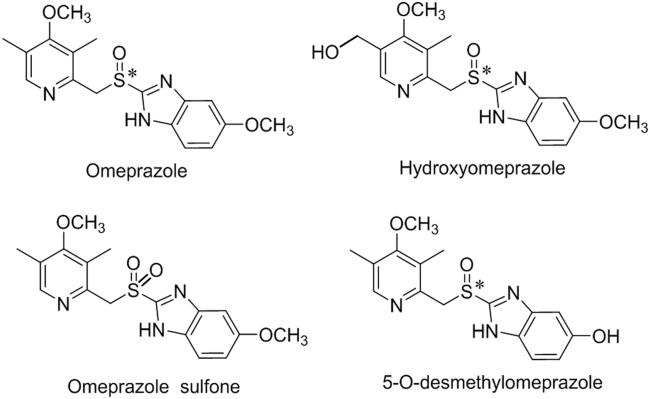
The substrate stereoselectivity in drug metabolism is exemplified by the metabolism of a proton pump inhibitor, omeprazole (Fig. 2). The asymmetric sulfur of omeprazole generates two enantiomeric forms, (S)- and (R)-omeprazole. Their main routes of metabolism, i.e., sulfoxidation and hydroxylation, have been shown to be mediated via CYP3A4 and CYP2C19, respectively [15], [16]. The predominant metabolism for the (S)-enantiomer is catalyzed by CYP3A4, which generates omeprazole sulfone. The (R)-enantiomer is metabolized primarily by CYP2C19, which generates hydroxyomeprazole and a minor metabolite, 5-O-desmethylomeprazole (Fig. 2) [24], [25]. As a consequence of the substrate stereoselective metabolism, the difference in oral bioavailability of two enantiomers is significant. As Abelö et al. [16] reported, the formation rate constant (intrinsic clearance) was 14.6 and 42.5 mL/min/mg protein for (S)- and (R)-omeprazole, respectively, which indicated that (S)-omeprazole was cleared more slowly than R-omeprazole in vivo. Thus, esomeprazole, the (S)-enantiomer of omeprazole was developed as an individual drug and has demonstrated significantly greater efficacy than omeprazole, while the tolerability and safety of esomeprazole were comparable to those of omeprazole [26].
Fig. 2.
The structure of omeprazole and its metabolites (the atom marked * is the chiral center).
Methylphenobarbital (MPB) (Fig. 3) is a drug for convulsions. The (R)-MPB was extensively hydroxylated, with an average of 49.56% of that enantiomer being converted to (R)-4-hydroxy-MPB, while only 7.16% of the (S)-MPB was converted to the corresponding hydroxy metabolite [27]. The extensive hydroxylation of (R)-MPB resulted in rapid elimination of this enantiomer, whereas the (S)-MPB was eliminated very slowly [27].
Fig. 3.
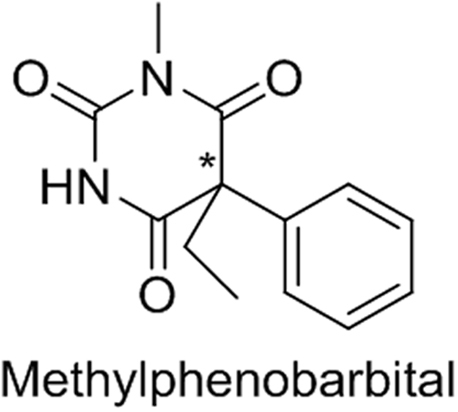
The structure of methylphenobarbital (the atom marked * is the chiral center).
2.2. Product stereoselectivity
Product stereoselectivity usually occurs in the substrates containing an element of prostereoisomerism (often a center of prochirality) during the reduction of ketone, the hydrogenation of carbon–carbon double bond, the oxygenation of prochiral substituent group, Phase II metabolic conjugated reaction, etc. [12]. During this metabolism, the prostereoisomerism is usually transformed into one configuration metabolite rather than the other. Therefore, although the drugs are achiral or pure enantiomer, their different configuration metabolites are produced at different rates by different enzymes. Considering interindividual variability or species differences of enzymes, product stereoselectivity may induce the different pharmacological activities in different people or species. As most of the drugs approved recently have a center of asymmetry and the majority are single enantiomers, product stereoselectivity in drug metabolism draws increasing attention.
Phenytoin (PPH, Fig. 4), a frequently prescribed drug for the treatment of epilepsy, possesses the prochiral para-hydroxylation. It is catalyzed by CYP2C9 and CYP2C19, and primarily metabolized to 5-(4′-hydroxyphenyl)-5-phenylhydantoin (p-HPPH), a mixture of (R)-p-HPPH and (S)-p-HPPH. As Argikar et al. [28], [29], [30] reported that the formation of HPPH from PPH was mediated exclusively by CYP2C9 and 2C19, with CYP2C9 playing the major role. PPH catalyzed by CYP2C9 was more inclined to form (S)-p-HPPH while PPH catalyzed by CYP2C19 did not have any tendency to form (R)-p-HPPH or (S)-p-HPPH. Therefore, the genetic polymorphisms of CYP2C9 and CYP2C19 will induce the different (S)/(R)-p-HPPH formation ratios.
Fig. 4.
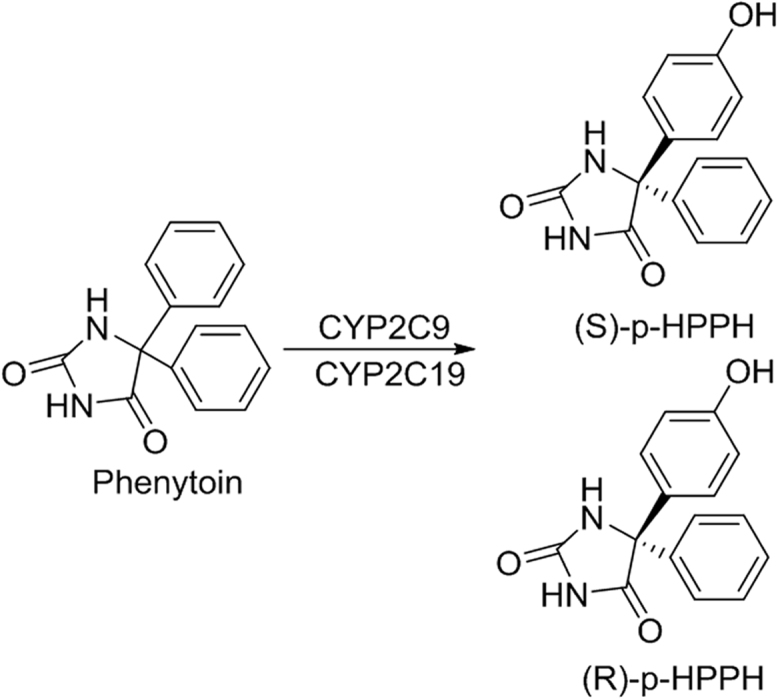
The structure of phenytoin and its metabolites.
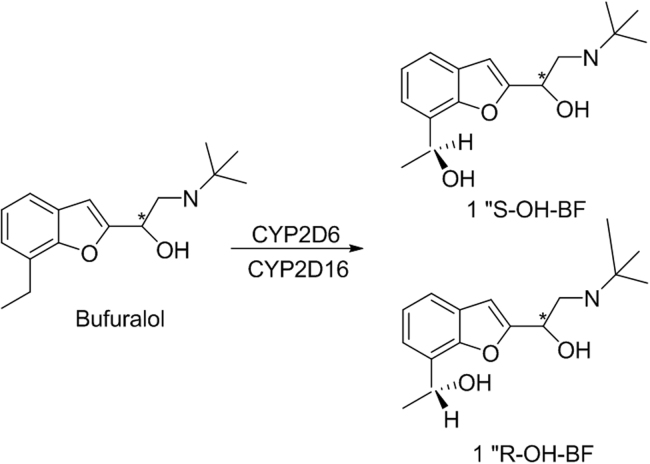
Bufuralol (BF, Fig. 5) is a nonselective β-adrenoceptor blocking agent, which has a chiral center in its molecule, yielding the eutomer, 1″S-BF, and the distomer, 1″R-BF. One of the metabolic pathways of BF is 1″-hydroxylation of an ethyl group, which introduces another chiral center into the BF molecule and yields four 1″-OH-BF diastereomers [31]. In ordinary human liver, this metabolic reaction shows a clear product stereoselectivity of 1″S-OH-BF>1″R-OH-BF from either BF enantiomers or racemates catalysed by the determinant enzyme CYP2D6. However, in poor CYP2D6 liver, it shows a product stereoselectivity of 1″S-OH-BF<1″R-OH-BF catalyzed by CYP2C19 (Fig. 5) [31]. Since there are different β-adrenoceptor blocking activities among the four 1″-OH-BF diastereomers, interindividual variability of pharmacological activities often occurs in different humans.
Fig. 5.
The structure of bufuralol and its metabolites.
2.3. Substrate–product stereoselectivity
As the name indicates, substrate–product stereoselectivity refers to the metabolism that contains both substrate stereoselectivity and product stereoselectivity, which means that the metabolites of racemates are more complex. Besides, substrate–product stereoselective metabolism contains chiral inversion, which makes no sense of producing single enantiomers.
A particularly instructive example of chiral inversion can be found in the metabolism of thalidomide (Fig. 6). Thalidomide has a chiral center and exists as racemates of (R)- and (S)-enantiomers. The (R)-enantiomer contains a sedative effect, while the (S)-enantiomer causes fetal malformations [32]. Scientists have made an attempt to separate the racemates to a pure enantiomer. However, whether animals were administered pure (R)-enantiomer or pure (S)-enantiomer, both hypnotic and teratogenic effects existed in animals, which suggested that single enantiomer may convert into the other in vivo [33]. Accordingly, there is no rationale for developing a single-enantiomer drug for thalidomide and other similar drugs.
Fig. 6.

The structure of thalidomide (the atom marked * is the chiral center).
3. Stereoselective analytical methods
The stereoselective metabolic process is so complex that only the methods of chiral recognition can make metabolic pathway clear. In order to know the extremely complex chiral inversion and chiral interactions, it is also necessary to establish a stereospecific method to analyze the individual enantiomer in biological samples such as plasma, urine, tissues and microsome.
As stereoselective analytical techniques have been developed, the accurate and reproducible determination of both drugs and metabolites in biological media has become possible. Recently, various methods are available for chiral bioanalytical assay, such as HPLC, GC, SFC, CE, NMR, and immunoassay. Among these techniques, chiral chromatography (HPLC, GC, SFC) and electrophoresis (CE) coupled with ultraviolet (UV), fluorescence (FL) and mass spectrometry (MS) detection are still the predominant analytical tools [34]. A comparison of these general techniques [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46] for the steroselective analysis of drugs and metabolites is presented in Table 2.
Table 2.
The comparison of general techniques for the steroselective analysis of drugs and their metabolites.
| Technique | Application | Complexity | Analysis speed | Efficiency | Cost | Ref. | |
|---|---|---|---|---|---|---|---|
| HPLC | CDR | Samples that have the functional group to be derivatized with | Moderate | Time consuming derivatization | Low | Low | [35], [36], [37] |
| CMPA | Samples that can generate diastereomer compounds with CMPAs | Simple | Long balance process in the column | Moderately high | Moderately high | ||
| CSP | Majority samples | Simple | Fast | High | High | ||
| GC | CDR | Samples that can be gasified | Complex | Slow | Low | Low | [38], [39] |
| CSP | Simple | Fast | High | High | |||
| SFC | Thermal instability and low volatile substances | Simple | Very fast | Very high | Low | [40], [41] | |
| CE | Majority samples | Simple | Very fast | High | Low | [42], [43] | |
| NMR | CDA | Samples that have groups reacted with CAD | Complex | Fast | High | Low | [44], [45] |
| LSR | Ester compounds | Simple | Fast | High | High | ||
| CSA | Alcohols, amines, carboxylic acids, etc. | Simple | Fast | High | Low | ||
| Immunoassay | Preliminary screening | Simple | Fast | High | – | [46] | |
CDR: Chiral derivatization reagent, CMPA: Chiral mobile phase additive, CSP: Chiral stationary phase, CDA: Chiral derivatizing agent, LSR: Lanthanide shift reagent, CSA: Chiral solvating agent.
3.1. HPLC
HPLC is still the most utilized technique for chiral separations in drug metabolism studies, which can be performed indirectly or directly [3], [47]. The indirect method is performed using chiral derivatization reagents (CDRs) while the direct method is performed by chiral mobile phase additives (CMPAs) or chiral stationary phases (CSPs) [3]. After the separation, enantiomers are usually detected by UV, FL or MS.
3.1.1. CSPs
CSPs is a preferred method due to its inherent advantages such as high speed, high reproducibility and flexibility during the analysis of enantiomers in complex mixtures [34], [48]. With the development of CSPs, many kinds of CSPs have been successfully used in the assays of chiral drugs and their metabolites in plasma, urine and liver microsomal fractions [34]. Currently, numerous commercial CSPs are available. According to their materials, CSPs can be divided into many types, such as proteins CSPs, amino acids CSPs, cyclodextrin CSPs, polysaccharide-based CSPs, crown ether CSPs, macrocyclic antibiotic CSPs, chiral ion- and ligand-exchange CSPs, and molecular imprinted polymer (MIP) CSPs. Polysaccharide-based CSPs and macrocyclic antibiotic CSPs are the most important and popular CSPs employed in HPLC [36], [47], [48].
3.1.1.1. Polysaccharide-based CSPs
Polysaccharide-based CSPs include polysaccharide derivatives CSPs (such as ChiralCel OJ, OA, OB, OK, CA-1), cellulose carbamate derivatives (such as ChiralCel OD, OC, OG, OF) and amylose CSPs (such as ChiralPak AD, AS) [49], [50]. Polysaccharide-based CSPs can be divided into coated and immobilized columns, which may exhibit different selectivities, because the conformation of the polymer is influenced by how the stationary phase is attached to the packing material [51]. Polysaccharide-based CSPs have their own features and are used for separation of different types of racemic compounds [52]. The polysaccharide-based CSPs have many important advantages such as large loading sample size, their ease to be superseded and functionalized, and a wide range of applications which have earned polysaccharide-based CSPs a great reputation in the field of chiral resolution [53]. Recently, an innovative method for the simultaneous determination of guaifenesin and ketorolac tromethamine enantiomers in plasma samples has been developed using amylose-2 as its chiral selector under the normal phase mode and using ornidazole as its internal standard [53]. This method is easy, reliable, and can be applied to the simultaneous quantitative analysis of drug enantiomers in other clinical samples. Yu et al. [54] developed a method to separate and quantify the enantiomers of a new potent selective 5-HT1B/1D receptor partial agonist, (S)-zolmitriptan, and its antipode in rat liver microsomes using a narrow-bore enantioselective normal phase Chiralpak AD-H column (250 mm×0.46 mm) with hexane–isopropanol–triethylamine as mobile phase and fluorescence detection. However, it should be noted that we should not use the eluents containing hydrochloric ethers, which will strip cellulose from the surface of silicon.
3.1.1.2. Macrocyclic antibiotic CSPs
Macrocyclic antibiotic CSPs include macrocyclic glycoprotein CSPs (such as teicoplanin, vancomycin) and ansamycin CSPs. Compared with other antibiotics, macrocyclic glycopeptides are more popular because of their unique versatility and broad selectivity [51]. Macrocyclic glycopeptides have multiple stereogenic centers and various functional groups which can provide stereoselective interactions with enantiomers [55]. Hefnawy et al. [56] developed and validated a method to determine the enantiomers of bisoprolol in human plasma using a teicoplanin macrocyclic antibiotic CSP known as Chirobiotic T with a polar ionic mobile phase consisting of methanol–glacial acetic acid–triethylamine and fluorescence detection. The human plasma sample was treated with solid phase extraction prior to HPLC analysis. Compared with Suzuki's method [57] achieved by a ChiralCel OD column and fluorescence detection, Hefnawy's method took less analysis time and gained a higher percentage of recovery from biological samples.
3.1.1.3. MIP CSPs and new CSPs
MIP is a material with high selectivity for specific molecules. It is made using a promising technique, molecular imprinting. Specifically, MIP is a specific artificial receptor that is prepared by a polymerization reaction of a template molecule (target molecule) and a functional monomer(s) followed by removing the template molecules to leave behind the permanent template grooves [58]. MIPs show high affinity and selectivity for their template molecule and have higher physical strength, robustness, resistance to elevated pressure and temperature, and inertness against various chemicals compared to biological media such as proteins and nucleic acids [58]. Therefore, when an enantiomer is used as the template, the produced MIP will show capability of enantiomeric recognition between the pair of enantiomers [59]. Due to these excellent properties, MIP has been largely used in chiral separations as stationary phases for HPLC, capillary electro chromatography, etc. [60], [61], [62]. However, no literature reports that MIP CSPs are used in assessing stereoselectivity in drug metabolism.
In addition, novel CSPs continue to be introduced to the market, such as the zwitterionic phases and new immobilized crown-ether phases [63], [64], [65]. Geditz et al. [66] successfully separated mefloquine (+)- and (−)-enantiomers and the carboxy metabolite in dried blood spots using a quinidine-based zwitterionic chiral stationary phase column, CHIRALPAK® ZWIX(−). However, chiral HPLC still lacks a CSP that can operate in all modes for separation of enantiomer molecules with different properties. Besides, due to the emergence of ultra high-performance liquid chromatography, small grain sizes of CSPs are also worth noting in the future [67].
3.1.2. Chiral mobile phase additives (CMPAs)
Although the method of chiral stationary phase has inherent advantages, it does cost more because it requires an expensive chiral column, which cannot be universally used due to its strict stereospecificity [68]. CMPAs can provide a simple and economical method for the chiral separation in some cases, which employs a conventional achiral column and mobile phase with a chiral selector [69]. During the separation, the chiral additive interacts with the enantiomers stereoselectively to achieve separation. The CMPAs method is more versatile, cheaper, and more flexible for enantioseparations [68].
At present, a large number of CMPAs have been used in the separation of chiral drugs. According to their separation mechanisms and structures, CMPAs can be divided into several types: ligand-exchangers, macrocyclic antibiotics, cyclodextrins, etc. [69]. Hatami and Farhadi [70] successfully separated ketoprofen enantiomers in human and rat plasma using hollow-fiber-based liquid-phase microextraction for sample treatment and vancomycin as a CMPA with an achiral C8 column.
However, the CMPAs method also has many drawbacks. First, the method is only suitable for limited compounds that can generate diastereomer with CMPAs. Second, the additive may interfere with the detection, which may affect the analysis result. Third, the balance process in the column is time-consuming and additive-consuming.
3.1.3. Chiral derivatization reagents (CDRs)
Some chiral drugs present such weak chromatographic properties on CSP that they cannot be separated by direct method. In these cases, CDRs may synthetically modify those drugs in order to enhance the chromatographic signal or increase the enantioselectivity. The derivatization usually reduces the polarity of the target molecule and increases the molecular mass and hydrophobicity of the relatively small molecules in order to prevent co-elution with high polar endogenous materials in complex matrices such as plasma and urine [71], [72].
A suitable CDR has to possess the following characteristics [71], [73]: (i) It should not cause any rearrangements or structural alterations of compounds during the formation of the derivative; (ii) The reagent should produce more than 95% complete derivatives; (iii) It should not contribute to loss of enantiomer during the reaction; (iv) It should produce a derivative that is stable and does not interact with the column. Currently, various kinds of CDRs are available, such as acylating reagents, thiocyanic acid, o-phthalaldehyde, esters reagents, etc. Nagao et al. [71] used novel chiral derivatization reagents (PyT-C and PyT-N) for chiral metabolomics identification by HPLC and electrospray-ionization MS. The proposed procedure using PyT-C and PyT-N has been applied to human saliva detections.
However, CDRs development is still in progress. In addition, the indirect method is applicable for some specific samples with functional groups to be derivatized or form complex with a chiral selector. Moreover, the derivatization represents an additional time consuming step and it has to be checked that no racemization takes place under the reaction conditions.
3.1.4. HPLC with mass spectrometry (LC/MS)
MS has become a popular analytical tool because of its high sensitivity and specificity compared to other detection techniques such as UV and FL [71]. Chiral drugs and their metabolites are usually at low levels in complex biological samples such as blood, tissues and urine, so more selective and sensitive methods are needed in their determination. Since the successful application of atmospheric pressure ionization (API) and the advances of MS, the combination of LC/MS has shown a significant impact on metabolite identification, which supports drug metabolism studies of chiral pharmaceuticals [74]. Nowadays, LC/MS has been widely used in studies on chiral drug metabolism [75].
API is the most utilized ionization mode, including electrospray ionization (ESI), atmospheric pressure chemical ionization (APCI), and atmospheric pressure photoionization (APPI). API is a soft ionization technique that only produces molecular ion peak and can determine drugs in mixture directly. ESI is widely used in the analysis of compounds that are polar, thermally unstable, or difficult to be gasified [38], [76]. Mass analyzer is the core of the mass spectrometer. LC combined with quadrupole analyzers, ion-trap analyzers, or high-resolution detectors such as time of flight (TOF) or Orbitrap analyzers becomes increasingly important in bioanalysis [33], [77], [78]. Ion-trap analyzers are useful in the qualitative analysis of complex compounds [79], [80]. TOF analyzers are suitable for analyzing biological macromolecules such as protein [81], [82]. Orbitrap analyzers coupled to reversed-phase LC represent the most ubiquitous approach to both small molecule and proteomic analyses [78], [83], [84], [85].
Stable isotope labeled compound is popularly used as internal standard in LC/MS. When one of the enantiomers is labeled by the isotope, the enantiomers can be discriminated via MS on the basis of their different molecular weights, regardless if they are separated on a column or not. Yu et al. [86] developed a sensitive and high-throughput HPLC–tandem mass spectrometry (LC–MS/MS) method coupled with stable isotope labeling technology. This method can simultaneously determine fluoxetine and norfluoxetine enantiomers in a CYP2C9 incubation mixture and has been used to study the metabolic interactions between fluoxetine enantiomers catalyzed by CYP2C9.
However, one of the most common problems for LC/MS encountered is the presence of matrix interference, which is usually difficult to eliminate by adjusting HPLC conditions, modifying sample preparation, or adding diethylamine or triethylamine [33].
3.2. Gas chromatography (GC)
Chiral GC, an important branch of chiral chromatography, can be divided into CDRs and CSPs. Both methods are established for applications based on the difference of the properties of enantiomers to achieve the separation.
GC with CSP is one of the important enantiomer-separating approaches and utilized extensively. There are various types of GC CSPs, such as peptide CSPs, bis-amides CSPs, chiral crown ether CSPs, cyclodextrins CSPs, cellulose CSPs, and chiral-metal CSPs [87], [88]. Among these CSPs, cyclodextrins CSPs are the most widely used CSPs in GC [35]. In recent years, GC CSPs have made some progress. Chiral ionic liquids, a new type of GC CSP, has been designed and utilized in the separation of chiral drugs based on properties such as nonflammability, high thermal stabilities, and variable polarities [89], [90]. Existing literature has reported that charged cyclodextrins as the chiral selectors resulted in significantly increased enantioselectivity and efficiency [91], [92], but no literature reports that these new types of GC CSPs can be used in the analysis of chiral drug metabolism. However, the use of ionic liquids for the analysis of chiral drug metabolism has become one of the developmental frontiers.
In addition to the same requirements for CDRs and its derivatization reactions of HPLC, CDRs and the generated corresponding derivatives of GC are also required to be volatile to apply to GC separation [42]. The commonly used chiral derivatization reagents contain carboxylic acid derivatives, amines, alcohols, isothiocyanates, etc. Tao et al. [93] developed a method for separating chiral phenethylamine agents on an achiral capillary gas chromatography by pre-column chiral derivatization with S-(−)-N-(fluoroacyl)-prolyl chloride. This method is simple, flexible, and economical for the analysis of chiral amine drug enantiomers in biological fluids and has been used to determine S-(+)-methamphetamine in human forensic samples and analyze enantiomers of amphetamine and fenfluramine in rat liver microsomes [93].
Like HPLC, GC/MS is widely used in studies on chiral drug metabolism and the advantages of GC/MS coupled with stable isotope labeling technology have been increasingly recognized [94].
3.3. Supercritical fluid chromatography (SFC)
Supercritical fluid is a material at a temperature and pressure above its critical point. SFC is a kind of chiral chromatographic technique using supercritical fluid as the mobile phase. CO2 is chiefly used due to its high diffusivity and low viscosity. In addition, the solvation strength of CO2 can be enhanced by the addition of polar organic modifiers [41], [95].
Although HPLC remains a main choice for enantio-separations and other separation techniques develop rapidly, SFC has become a better choice, especially in bioanalysis, due to its fast analysis speed, wide polarity compatibility, high column efficiency, low cost, and environment-friendly mobile phase [96]. Recently, a new method for the determination of tebuconazole in water and zebrafish was achieved by SFC-MS/MS [97]. Its mobile phase was supercritical CO2 with a small amount of methanol, which is simple, low in toxicity, and environmentally friendly. Its flow rate was 2.0 mL/min and its analysis time was much shorter than that of HPLC. Moreover, CSPs of SFC were developed based on CSPs of HPLC and GC, and many CSPs can be used for SFC without any pretreatment [98]. In some cases, tandem arrangements (chiral–chiral columns or achiral–chiral columns) can even increase the total column efficiency and column capacity, which is beneficial for the separation of enantiomers in multicomponent mixture [99], [100].
However, despite the progress in SFC for chiral separation and determination, researchers still have relatively less interest in SFC [40]. The main reason is that SFC lacks robustness and repeatability. Moreover, though the addition of an organic modifier in the mobile phase (possibly with the addition of a third component at low concentration) may afford elution of polar components, SFC is not suitable for very polar drug [40].
3.4. Capillary electrophoresis (CE)
CE separation depends on the differential migration of solutes in an electric field and the driving forces are electrophoretic migration and electro-osmotic flow [101]. CE has incomparable advantages in chiral drugs and their metabolites analysis. It can analyze a drug and its phase II metabolites simultaneously and presents the outstanding advantages of high resolving capability and minimum solvent and reagent consumption, which is suitable for analysis of small samples, such as infant blood samples and in vivo microdialysis samples [102], [103].
Similar to HPLC, CE prefers to employ CSPs as chiral selectors, such as cyclodextrins, polysaccharides derivatives, macrocyclic antibiotics and proteins. The detection of CE is usually achieved by UV. Hamidi et al. [104] developed a method for chiral separation of carvedilol in human plasma using CE coupled to ultraviolet diode array detection. However, the sensitivity of UV is low because the signal is directly related to an optical path length afforded by an internal diameter of a capillary [105]. Thus, CE/MS has been regarded as a useful complementary analytical technique for the profiling of (highly) polar ionogenic metabolites in biological samples, especially in the field of metabolomics [106]. A method for identifying ecstasy and methadone in plasma was developed using CE-electrospray ionization mass spectrometry, which was successfully applied to quantitation of ecstasy and methadone in real cases [107].
However, the surfactants in micellar electrokinetic chromatography and microemulsion electrokinetic chromatography, which are used for the separation of neutral species, can cause several problems in MS, including ion source contamination and baseline noise [108]. CE still needs to be changed in many ways, and the majority of these changes must be made to the chiral columns. The repeatability of different capillary columns is one of the problems hindering the development of the CE.
3.5. NMR
With the rapid development of high-resolution NMR instruments, NMR has become a very useful tool in many fields. In the study of chiral drugs, methods for determining the absolute configuration of chiral compounds require the structure parameters of enantiomers, which can be acquired from NMR atlas, such as the chemical shifts, the coupling constants, the relaxation rates, and the areas of NMR absorption peak [109].
However, NMR spectroscopy fails to distinguish enantiomers in the conventional achiral solvents because of the identical magnetic environment [44]. In order to use NMR in chiral analysis, enantiomers must be converted to diastereomers, which permits the enantio-discrimination and the precise measurement of enantiomeric excess [110]. Depending on the type of chiral auxiliary reagents, three major methods can be used to determine enantiomers. The first applies chiral derivatizing agents (CDAs), which can transform enantiomers into stable diastereomeric derivatives [111]. The second uses the chiral lanthanide shift reagent (LSRs), which can interact with enantiomers and form coordination compounds [112]. The third employs certain chiral solvating agents (CSAs), which can transform enantiomers into diamagnetic and instable diastereomers [113]. The first is an indirect method while the others are direct methods.
CDAs are kinds of enantiomerically pure agents, such as (S)-methoxyphenylacetic acid, phosphorus CDA, and organometallic CDA. Compared with LSR or CSA, CDA has a more stationary nuclear magnetic signal [44], [45]. However, there are more requirements for CDA. First, derivatizing agents should be optically pure, which is not required for the method of CSA. Second, no racemization or kinetic resolution occurs during derivatization, while racemization or kinetic resolution would not accrue in the method of CSA. Third, the purification of products can only use the non-selective enrichment method. Therefore, CDAs are usually used in the determination of enantiomeric purity in synthetic compounds, but rarely applied to stereoselectivity assessing in drug metabolism. LSRs are more widely used in the determination of ester compounds than CDAs or CSAs because the ester compounds need not be hydrolyzed before determination in LSR. However, most LSRs cause excessive broadening of NMR peaks of chiral substrates [44]. CSAs are more attractive in the analysis of chiral drugs and their metabolites because the method of CSA usually does not suffer from the broadening of the signals in the NMR spectrum and the test enantiomers need not be derivatized and/or purified prior to analysis. Pérez-Trujillo et al. [114] developed an 1H NMR-based method for the differentiation and identification of chiral drug enantiomers (two enantiomers and one metabolite of ibuprofen) in human urine via direct cosolvation with β-cyclodextrin. This method does not require any pretreatment and has the potential to be adapted for determining other enantio-specific metabolic profilings.
Furthermore, NMR can also be used in structure elucidation. In pharmacological drug metabolite studies, the need for de novo structure elucidation is becoming more frequently, thus, LC-NMR, CE-NMR and other coupling techniques can be very convenient [111], [115]. Su et al. [116] elucidated and identified five phase I metabolites and ten phase II metabolites of isobavachalcone in rat bile using LC-ESI-MSn and LC-NMR method, because simultaneously using LC-MS and LC-NMR can supply valuable information on chemical structure and molecular weight of compounds [117].
3.6. Immunoassay
Immunoassay is based on the specific binding reaction between antigen and antibody (Ab). It is usually used in qualitative and quantitative analysis of biological samples, such as blood, saliva, urine, as well as hormones and cytokines. As Abs are chiral molecules and the binding between chiral drugs and Abs may involve stereoselective interactions, Abs have the potential to split drug enantiomers [118].
Stereoselective immunoassay is becoming increasingly important in pharmaceutical analysis, especially in chiral analysis. There are many types of stereoselective immunoassays, such as radioimmunoassay, enzyme-linked immunosorbent assay, and fluorescent immunoassay [119]. Stereoselective immunoassay is sensitive, specific and fast [120]. Once the method is established, a large number of samples can be measured conveniently. What is more, due to the high specificity of the antigen–antibody reaction, the enantiomers can be determined directly from complex biological samples [121].
However, stereoselective immunoassay is largely neglected in the application of chiral drugs analysis. The rapid development of chiral chromatography may be one of the reasons, but the drawbacks of immunoassay are likely the main reasons. Since each chiral drug needs a specific antibody, the development of a new immunoassay method becomes complex and time-consuming. To develop a new enantioselective immunoassay, high stereoselective Abs must be available at first. For the different chiral centers of chiral compounds, different chiral Abs need to be prepared and their ability of chiral recognition needs to be evaluated, and then the immune-reagent needs to be designed. However, it is still primarily based on the immunochemist's experiences and a “trial and error” approach due to the lack of understanding of chiral interactions between haptens and antibodies [46]. Antibodies may react with compounds with structures similar to target compounds, such as drug metabolites. If these issues can be overcome, immunoassay is expected to be applied to preliminary screening and clinical tests.
4. Conclusion
Metabolism is the most important process in drug disposition and is the most relevant process in stereoselectivity [1], [122]. Therefore, stereoselective metabolic pathways influence the pharmacological activities, tolerability, safety, and bioavailability of drugs directly. Chiral inversion, a type of substrate–product stereoselectivity, is a phenomenon worth noting, especially when one of the enantiomers has significant toxicity and side effects. In addition, due to the participation of enzymes, enantiomers catalyzed by different enzymes can cause different kinds of drug–drug interactions. However, the stereoselective metabolic process is so complex that only the methods of chiral recognition can make metabolic pathway clear. Consequently, stereoselectivity assessing methods in drug metabolism are of great relevance to pharmaceutical R&D and the rational use in clinic.
Nowadays, various methods are used for stereoselectivity assessing in drug metabolism. Chromatographic methods are still the most popular techniques, including HPLC, GC, SFC, and CE. The limitations of indirect methods are that they are applicable to some specific samples with functional groups to be derivatized or form complex with a chiral selector [62], [64]. Direct methods achieved by CSPs are used more frequently due to their elegant and simple approaches, but CSPs are expensive. Furthermore, CSPs of CE have poor repeatability, which has been one of the problems hindering the development of CE. Consequently, high selectivity and universal CSPs still need to be developed. Chromatographic techniques are usually coupled to UV, FL and MS. However, chiral drugs and their metabolites are usually at too low a concentration in complex biological samples to meet the detection limits of UV or FL. Although MS is highly sensitive and specific, the matrix interference is still a challenge to overcome.
In addition, NMR and immunoassay used in chiral analysis are worth noting. For example, NMR can be used in structure elucidation, which is useful in analyzing metabolites with unknown structures. Immunoassay is expected to be applied to preliminary screening and clinical tests, if the problem of how to establish a new enantioselective immunoassay simply can be solved. Additionally, recombinant antibodies may improve the repeatability of immunoassay.
Compared with typical achiral assessing methods, although stereoselectivity assessing methods in drug metabolism face more challenges, the optimization of existing techniques or tandem techniques may solve these problems.
Acknowledgments
We acknowledge financial support from the National Major Projects of China (2011CB710800), the International Science & Technology Cooperation Program of China (2014DFE30050), and the Program for Zhejiang Leading Team of S&T Innovation (2011R50014). We also show thanks to Dr. Ni Ai for the helpful discussion.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Rentsch K.M. The importance of stereoselective determination of drugs in the clinical laboratory. J. Biochem. Biophys. Methods. 2002;54:1–9. doi: 10.1016/s0165-022x(02)00124-0. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen L.A., He H., Pham-Huy C. Chiral drugs: an overview. Int. J. Biomed. Sci. 2006;2:85–100. [PMC free article] [PubMed] [Google Scholar]
- 3.Campo V.L., Bernardes L.S., Carvalho I. Stereoselectivity in drug metabolism: molecular mechanisms and analytical methods. Curr. Drug Metab. 2009;10:188–205. doi: 10.2174/138920009787522188. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y., Cao J., Wang X. Stereoselective transport and uptake of propranolol across human intestinal Caco-2 cell monolayers. Chirality. 2010;22:361–368. doi: 10.1002/chir.20753. [DOI] [PubMed] [Google Scholar]
- 5.Shen Q., Wang L., Zhou H. Stereoselective binding of chiral drugs to plasma proteins. Acta Pharmacol. Sin. 2013;34:998–1006. doi: 10.1038/aps.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolfe M.S. Inhibition and modulation of γ-secretase for Alzheimer's disease. Neurotherapeutics. 2008;5:391–398. doi: 10.1016/j.nurt.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kukar T., Prescott S., Eriksen J.L. Chronic administration of R-flurbiprofen attenuates learning impairments in transgenic amyloid precursor protein mice. BMC Neurosci. 2007;8:54. doi: 10.1186/1471-2202-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agranat I., Wainschtein S.R., Zusman E.Z. The predicated demise of racemic new molecular entities is an exaggeration. Nat. Rev. Drug Discov. 2012;11:972–973. doi: 10.1038/nrd3657-c1. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Food and Drug Administration, Compilation Prepared by U.S. Department of Health and Human Services, 2011-2014. 〈http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugInnovation/default.htm〉.
- 10.Niwa T., Murayama N., Yamazaki H. Stereoselectivity of human cytochrome p450 in metabolic and inhibitory activities. Curr. Drug Metab. 2011;12:549–569. doi: 10.2174/138920011795713724. [DOI] [PubMed] [Google Scholar]
- 11.Rowland A., Miners J.O., Mackenzie P.I. The UDP-glucuronosyltransferases: their role in drug metabolism and detoxification. Int. J. Biochem. Cell Biol. 2013;45:1121–1132. doi: 10.1016/j.biocel.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 12.Testa B. Types of stereoselectivity in drug metabolism: a heuristic approach. Drug Metab. Rev. 2015;47:239–251. doi: 10.3109/03602532.2014.984814. [DOI] [PubMed] [Google Scholar]
- 13.Chen C.S., Jounaidi Y., Waxman D.J. Enantioselective metabolism and cytotoxicity of R-ifosfamide and S-ifosfamide by tumor cell-expressed cytochromes P450. Drug Metab. Dispos. 2005;33:1261–1267. doi: 10.1124/dmd.105.004788. [DOI] [PubMed] [Google Scholar]
- 14.Totah R.A., Allen K.E., Sheffels P. Enantiomeric metabolic interactions and stereoselective human methadone metabolism. J. Pharmacol. Exp. Ther. 2007;321:389–399. doi: 10.1124/jpet.106.117580. [DOI] [PubMed] [Google Scholar]
- 15.Li X.Q., Weidolf L., Simonsson R. Enantiomer/enantiomer interactions between the S-and R-isomers of omeprazole in human cytochrome P450 enzymes: major role of CYP2C19 and CYP3A4. J. Pharmacol. Exp. Ther. 2005;315:777–787. doi: 10.1124/jpet.105.090928. [DOI] [PubMed] [Google Scholar]
- 16.Abelö A., Andersson T.B., Antonsson M. Stereoselective metabolism of omeprazole by human cytochrome P450 enzymes. Drug Metab. Dispos. 2000;28:966–972. [PubMed] [Google Scholar]
- 17.Thijssen H.H., Flinois J.P., Beaune P.H. Cytochrome P4502C9 is the principal catalyst of racemic acenocoumarol hydroxylation reactions in human liver microsomes. Drug Metab. Dispos. 2000;28:1284–1290. [PubMed] [Google Scholar]
- 18.Shen L., Fitzloff J.F., Cook C.S. Differential enantioselectivity and product-dependent activation and inhibition in metabolism of verapamil by human CYP3As. Drug Metab. Dispos. 2004;32:186–196. doi: 10.1124/dmd.32.2.186. [DOI] [PubMed] [Google Scholar]
- 19.Tracy T.S., Korzekwa K.R., Gonzalez F.J. Cytochrome P450 isoforms involved in metabolism of the enantiomers of verapamil and norverapamil. Br. J. Clin. Pharmacol. 1999;47:545–552. doi: 10.1046/j.1365-2125.1999.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mautz D.S., Shen D.D., Nelson W.L. Regioselectivity and enantioselectivity of metoprolol oxidation by two variants of cDNA-expressed P4502D6. Pharm. Res. 1995;12:2053–2056. doi: 10.1023/a:1016233115443. [DOI] [PubMed] [Google Scholar]
- 21.Ufer M., Kammerer B., Kahlich R. Genetic polymorphisms of cytochrome P450 2C9 causing reduced phenprocoumon (S)-7-hydroxylation in vitro and in vivo. Xenobiotica. 2004;34:847–859. doi: 10.1080/00498250400009197. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M., Fawcett J.P., Kennedy J.M. Stereoselective glucuronidation of formoterol by human liver microsomes. Br. J. Clin. Pharmacol. 2000;49:152–157. doi: 10.1046/j.1365-2125.2000.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walle U., Pesola G., Walle T. Stereoselective sulphate conjugation of salbutamol in humans: comparison of hepatic, intestinal and platelet activity. Br. J. Clin. Pharmacol. 1993;35:413–418. doi: 10.1111/j.1365-2125.1993.tb04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.VandenBranden M., Ring B.J., Binkley S.N. Interaction of human liver cytochromes P450 in vitro with LY307640, a gastric proton pump inhibitor. Pharmacogenetics. 1996;6:81–91. doi: 10.1097/00008571-199602000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Andersson T., Miners J.O., Veronese M.E. Identification of human liver cytochrome P450 isoforms mediating omeprazole metabolism. Br. J. Clin. Pharmacol. 1993;36:521–530. doi: 10.1111/j.1365-2125.1993.tb00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richter J.E., Kahrilas P.J., Johanson J. Efficacy and safety of esomeprazole compared with omeprazole in GERD patients with erosive esophagitis: a randomized controlled trial. Am. J. Gastroenterol. 2001;96:656–665. doi: 10.1111/j.1572-0241.2001.3600_b.x. [DOI] [PubMed] [Google Scholar]
- 27.Lim W., Hooper W.D. Stereoselective metabolism and pharmacokinetics of racemic methylphenobarbital in humans. Drug. Metab. Dispos. 1989;17:212–217. [PubMed] [Google Scholar]
- 28.Argikar U.A., Cloyd J.C., Birnbaum A.K. Paradoxical urinary phenytoin metabolite (S)/(R) ratios in CYP2C19* 1/* 2 patients. Epilepsy Res. 2006;71:54–63. doi: 10.1016/j.eplepsyres.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Giancarlo G.M., Venkatakrishnan K., Granda B.W. Relative contributions of CYP2C9 and 2C19 to phenytoin 4-hydroxylation in vitro: inhibition by sulfaphenazole, omeprazole, and ticlopidine. Eur. J. Clin. Pharmacol. 2001;57:31–36. doi: 10.1007/s002280100268. [DOI] [PubMed] [Google Scholar]
- 30.Caraco Y., Muszkat M., Wood A.J. Phenytoin metabolic ratio: a putative marker of CYP2C9 activity in vivo. Pharmacogenetics. 2001;11:587–596. doi: 10.1097/00008571-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Narimatsu S., Takemi C., Kuramoto S. Stereoselectivity in the oxidation of bufuralol, a chiral substrate, by human cytochrome P450s. Chirality A. 2003;5:333–339. doi: 10.1002/chir.10212. [DOI] [PubMed] [Google Scholar]
- 32.Höglund P., Eriksson T., Björkman S. A double-blind study of the sedative effects of the thalidomide enantiomers in humans. J. Pharmacokinet. Biopharm. 1998;26:363–383. doi: 10.1023/a:1021008016719. [DOI] [PubMed] [Google Scholar]
- 33.Kumar S., Rajkumar S.V. Thalidomide and lenalidomide in the treatment of multiple myeloma. Eur. J. Cancer. 2006;42:1612–1622. doi: 10.1016/j.ejca.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Bonato P.S. Research Spotlight: stereoselective analysis of drugs and metabolites by the Chromatographic and Electrophoretic Analysis Center group. Bioanalysis. 2010;2:175–179. doi: 10.4155/bio.09.161. [DOI] [PubMed] [Google Scholar]
- 35.Ward T.J., Ward K.D. Chiral separations: a review of current topics and trends. Anal. Chem. 2011;84:626–635. doi: 10.1021/ac202892w. [DOI] [PubMed] [Google Scholar]
- 36.G. Gübitz, M.G. Schmid, Chiral Separations, John Wiley and Sons, New York, 2004, pp. 183.
- 37.Chankvetadze B. Recent developments on polysaccharide-based chiral stationary phases for liquid-phase separation of enantiomers. J. Chromatogr. A. 2012;1269:26–51. doi: 10.1016/j.chroma.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 38.Halket J.M., Waterman D., Przyborowska A.M. Chemical derivatization and mass spectral libraries in metabolic profiling by GC/MS and LC/MS/MS. J. Exp. Bot. 2005;56:219–243. doi: 10.1093/jxb/eri069. [DOI] [PubMed] [Google Scholar]
- 39.Fujii H., Hara K., Kashiwagi M. Application of high-throughput chiral analysis of amphetamines by GC–MS to whole blood specimens. Forensic Toxicol. 2013;31:183–185. [Google Scholar]
- 40.Płotka J.M., Biziuk M., Morrison C. Pharmaceutical and forensic drug applications of chiral supercritical fluid chromatography. Trends Anal. Chem. 2014;56:74–89. [Google Scholar]
- 41.De Klerck K., Mangelings D., Vander Heyden Y. Supercritical fluid chromatography for the enantioseparation of pharmaceuticals. J. Pharm. Biomed. Anal. 2012;69:77–92. doi: 10.1016/j.jpba.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 42.Gübitz G., Schmid M.G. Chiral separation by capillary electromigration techniques. J. Chromatogr. A. 2008;1204:140–156. doi: 10.1016/j.chroma.2008.07.071. [DOI] [PubMed] [Google Scholar]
- 43.Hadley M.R., Camilleri P., Hutt A.J. Enantiospecific analysis by capillary electrophoresis: applications in drug metabolism and pharmacokinetics. Electrophoresis. 2000;21:1953–1976. doi: 10.1002/1522-2683(20000601)21:10<1953::AID-ELPS1953>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 44.Chaudhari S.R., Suryaprakash N. Recent NMR methodological developments for chiral analysis in isotropic solutions. J. Indian Inst. Sci. 2014;94:485–516. [Google Scholar]
- 45.G. Uccello-Barretta, F. Balzano, Chiral NMR Solvating Additives for Differentiation of Enantiomers, Springer, Berlin, 2013, pp. 69–131. [DOI] [PubMed]
- 46.Mu H., Wang B., Xu Z. Stereospecific recognition and quantitative structure–activity relationship between antibodies and enantiomers: ofloxacin as a model hapten. Analyst. 2015;140:1037–1045. doi: 10.1039/c4an02155j. [DOI] [PubMed] [Google Scholar]
- 47.Fortuna A., Alves G., Falcão A. Chiral chromatographic resolution of antiepileptic drugs and their metabolites: a challenge from the optimization to the application. Biomed. Chromatogr. 2014;28:27–58. doi: 10.1002/bmc.3004. [DOI] [PubMed] [Google Scholar]
- 48.Ward T.J., Ward K.D. Chiral separations: fundamental review 2010. Anal. Chem. 2010;82:4712–4722. doi: 10.1021/ac1010926. [DOI] [PubMed] [Google Scholar]
- 49.Yashima E. Polysaccharide-based chiral stationary phases for high-performance liquid chromatographic enantioseparation. J. Chromatogr. A. 2001;906:105–125. doi: 10.1016/s0021-9673(00)00501-x. [DOI] [PubMed] [Google Scholar]
- 50.Ma S., Tsui H.W., Spinelli E. Insights into chromatographic enantiomeric separation of allenes on cellulose carbamate stationary phase. J. Chromatogr. A. 2014;1362:119–128. doi: 10.1016/j.chroma.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 51.T.J. Ward, K.D. Ward, Recent Progress in Chiral Stationary Phase Development and Current Chiral Applications, 2014. 〈http://www.chromatographyonline.com/recent-progress-chiral-stationary-phase-development-and-current-chiral-applications〉.
- 52.Kennedy J.H. Comparison of chiral separations on polysaccharide chiral stationary phases to an improved Pirkle phase. J. Chromatogr. A. 1996;725:219–224. [Google Scholar]
- 53.Maher H.M., Al-Taweel S.M., Alshehri M.M. Novel stereoselective high-performance liquid chromatographic method for simultaneous determination of guaifenesin and ketorolac enantiomers in human plasma. Chirality. 2014;26:629–639. doi: 10.1002/chir.22354. [DOI] [PubMed] [Google Scholar]
- 54.Yu L., Yao T., Ni S. Determination of zolmitriptan enantiomers in rat liver microsomes by chiral high performance liquid chromatography with fluorescence detection. Biomed. Chromatogr. 2005;19:191–195. doi: 10.1002/bmc.433. [DOI] [PubMed] [Google Scholar]
- 55.Tesařová E., Záruba K., Flieger M. Enantioseparation of semisynthetic ergot alkaloids on vancomycin and teicoplanin stationary phases. J. Chromatogr. A. 1999;844:137–147. [Google Scholar]
- 56.Hefnawy M.M., Sulta M.M.A., Al-Shehri M.M. Enantioanalysis of bisoprolol in human plasma with a macrocyclic antibiotic HPLC chiral column using fluorescence detection and solid phase extraction. Chem. Pharm. Bull. 2007;55:227–230. doi: 10.1248/cpb.55.227. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki T., Horikiri Y., Mizobe M. Sensitive determination of bisoprolol enantiomers in plasma and urine by high-performance liquid chromatography using fluorescence detection, and application to preliminary study in humans. J. Chromatogr. B. 1993;619:267–273. doi: 10.1016/0378-4347(93)80116-l. [DOI] [PubMed] [Google Scholar]
- 58.Cheong W.J., Yang S.H., Ali F. Molecular imprinted polymers for separation science: a review of reviews. J. Sep. Sci. 2013;36:609–628. doi: 10.1002/jssc.201200784. [DOI] [PubMed] [Google Scholar]
- 59.Cheong W.J., Ali F., Choi J.H. Recent applications of molecular imprinted polymers for enantio-selective recognition. Talanta. 2013;106:45–59. doi: 10.1016/j.talanta.2012.11.049. [DOI] [PubMed] [Google Scholar]
- 60.W.J. Cheong, S.H. Yang, Open Tubular Molecular Imprinted Phases in Chiral Capillary Electrochromatography, Springer, Berlin, 2013, pp. 469–487. [DOI] [PubMed]
- 61.Kulsing C., Knob R., Macka M. Molecular imprinted polymeric porous layers in open tubular capillaries for chiral separations. J. Chromatogr. A. 2014;1354:85–91. doi: 10.1016/j.chroma.2014.05.065. [DOI] [PubMed] [Google Scholar]
- 62.Tang M., Zhang J., Zhuang S. Development of chiral stationary phases for high-performance liquid chromatographic separation. Trends Anal. Chem. 2012;39:180–194. [Google Scholar]
- 63.Wernisch S., Pell R., Lindner W. Increments to chiral recognition facilitating enantiomer separations of chiral acids, bases, and ampholytes using Cinchona‐based zwitterion exchanger chiral stationary phases. J. Sep. Sci. 2012;35:1560–1572. doi: 10.1002/jssc.201200103. [DOI] [PubMed] [Google Scholar]
- 64.Wang D., Zhao J., Wu H. Preparation and evaluation of novel chiral stationary phases based on quinine derivatives comprising crown ether moieties. J. Sep. Sci. 2015;38:205–210. doi: 10.1002/jssc.201400977. [DOI] [PubMed] [Google Scholar]
- 65.M.H. Hyun, Enantioseparations of Primary Amino Compounds by High-performance Liquid Chromatography Using Chiral Crown Ether-based Chiral Stationary Phase, Springer, Berlin, 2013, pp. 165–176. [DOI] [PubMed]
- 66.Geditz M.C., Lindner W., Lämmerhofer M. Simultaneous quantification of mefloquine (+)-and (−)-enantiomers and the carboxy metabolite in dried blood spots by liquid chromatography/tandem mass spectrometry. J. Chromatogr. B. 2014;968:32–39. doi: 10.1016/j.jchromb.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 67.Novakova L., Matysova L., Solich P. Advantages of application of UPLC in pharmaceutical analysis. Talanta. 2006;68:908–918. doi: 10.1016/j.talanta.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 68.Tong S., Zhang H., Shen M. Enantioseparation of mandelic acid derivatives by high performance liquid chromatography with substituted beta-cyclodextrin as chiral mobile phase additive and evaluation of inclusion complex formation. J. Chromatogr. B. 2014;962:44–51. doi: 10.1016/j.jchromb.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.L. Yu, S. Wang, S. Zeng, Chiral Mobile Phase Additives in HPLC Enantioseparations, Springer, Berlin, 2013, pp. 221–231. [DOI] [PubMed]
- 70.Hatami M., Farhadi K. Analysis of ketoprofen enantiomers in human and rat plasma by hollow-fiber-based liquid-phase microextraction and chiral mobile-phase additive HPLC. Can. J. Chem. 2013;91:1252–1257. [Google Scholar]
- 71.Nagao R., Tsutsui H., Mochizuki T. Novel chiral derivatization reagents possessing a pyridylthiourea structure for enantiospecific determination of amines and carboxylic acids in high-throughput liquid chromatography and electrospray-ionization mass spectrometry for chiral metabolomics identification. J. Chromatogr. A. 2013;1296:111–118. doi: 10.1016/j.chroma.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 72.Toyo’oka T. Resolution of chiral drugs by liquid chromatography based upon diastereomer formation with chiral derivatization reagents. J. Biochem. Biophys. Methods. 2002;54:25–56. doi: 10.1016/s0165-022x(02)00127-6. [DOI] [PubMed] [Google Scholar]
- 73.F. Orata, Derivatization Reactions and Reagents for Gas Chromatography Analysis, INTECH Open Access Publisher, Croatia, 2012, pp. 83–108.
- 74.Peng L., Jayapalan S., Chankvetadze B. Reversed-phase chiral HPLC and LC/MS analysis with tris (chloromethylphenylcarbamate) derivatives of cellulose and amylose as chiral stationary phases. J. Chromatogr. A. 2010;1217:6942–6955. doi: 10.1016/j.chroma.2010.08.075. [DOI] [PubMed] [Google Scholar]
- 75.Li W., Zhao L., Le J. Evaluation of tetrahydropalmatine enantiomers on the activity of five cytochrome P450 isozymes in rats using a liquid chromatography/mass spectrometric method and a cocktail approach. Chirality. 2015;27:551–556. doi: 10.1002/chir.22469. [DOI] [PubMed] [Google Scholar]
- 76.Strege M.A. High-performance liquid chromatographic-electrospray ionization mass spectrometric analyses for the integration of natural products with modern high-throughput screening. J. Chromatogr. B. 1999;725:67–78. doi: 10.1016/s0378-4347(98)00553-2. [DOI] [PubMed] [Google Scholar]
- 77.Rochat B., Kottelat E., McMullen J. The future key role of LC-high-resolution-MS analyses in clinical laboratories: a focus on quantification. Bioanalysis. 2012;4:2939–2958. doi: 10.4155/bio.12.243. [DOI] [PubMed] [Google Scholar]
- 78.Scigelova M., Makarov A. Orbitrap mass analyzer – overview and applications in proteomics. Proteomics. 2006;6:16–21. doi: 10.1002/pmic.200600528. [DOI] [PubMed] [Google Scholar]
- 79.Karu K., Hornshaw M., Woffendin G. Liquid chromatography–mass spectrometry utilizing multi-stage fragmentation for the identification of oxysterols. J. Lipid Res. 2007;48:976–987. doi: 10.1194/jlr.M600497-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hopfgartner G., Varesio E., Tschäppät V. Triple quadrupole linear ion trap mass spectrometer for the analysis of small molecules and macromolecules. J. Mass Spectrom. 2004;39:845–855. doi: 10.1002/jms.659. [DOI] [PubMed] [Google Scholar]
- 81.Cotter R.J. Time-of-flight mass spectrometry for the structural analysis of biological molecules. Anal. Chem. 1992;64:1027A–1039A. doi: 10.1021/ac00045a002. [DOI] [PubMed] [Google Scholar]
- 82.Svergun D.I., Koch M.H. Small-angle scattering studies of biological macromolecules in solution. Rep. Prog. Phys. 2003;66:1735. doi: 10.1017/s0033583503003871. [DOI] [PubMed] [Google Scholar]
- 83.Makarov A., Scigelova M. Coupling liquid chromatography to Orbitrap mass spectrometry. J. Chromatogr. A. 2010;1217:3938–3945. doi: 10.1016/j.chroma.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 84.Hu Q., Nol R.J., Li H. The Orbitrap: a new mass spectrometer. J. Mass Spectrom. 2005;40:430–443. doi: 10.1002/jms.856. [DOI] [PubMed] [Google Scholar]
- 85.Lu W., Clasquin M.F., Melamud E. Metabolomic analysis via reversed-phase ion-pairing liquid chromatography coupled to a stand alone orbitrap mass spectrometer. Anal. Chem. 2010;82:3212–3221. doi: 10.1021/ac902837x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu L., Wang S., Jiang H. Simultaneous determination of fluoxetine and norfluoxetine enantiomers using isotope discrimination mass spectroscopy solution method and its application in the CYP2C9-mediated stereoselective interactions. J. Chromatogr. A. 2012;1236:97–104. doi: 10.1016/j.chroma.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 87.Pirkle W., Pochapsky T. Separation of the stereoisomers of a homologeous series of bis-amides on chiral stationary phases. Chromatographia. 1988;25:652–654. [Google Scholar]
- 88.Berthod A., Li W., Armstrong D.W. Multiple enantioselective retention mechanisms on derivatized cyclodextrin gas chromatographic chiral stationary phases. Anal. Chem. 1992;64:873–879. [Google Scholar]
- 89.Sun X., Xu J., Zhao X. Study of chiral ionic liquid as stationary phases for GC. Chromatographia. 2013;76:1013–1019. [Google Scholar]
- 90.Baudequin C., Brégeon D., Levillain J. Chiral ionic liquids, a renewal for the chemistry of chiral solvents? Design, synthesis and applications for chiral recognition and asymmetric synthesis. Tetrahedron: Asymmetry. 2005;16:3921–3945. [Google Scholar]
- 91.Beneš M., Zusková I., Svobodová J. Determination of stability constants of complexes of neutral analytes with charged cyclodextrins by affinity capillary electrophoresis. Electrophoresis. 2012;33:1032–1039. doi: 10.1002/elps.201100489. [DOI] [PubMed] [Google Scholar]
- 92.A.C. Servais, M. Fillet, Enantioseparations in Nonaqueous Capillary Electrophoresis Using Charged Cyclodextrins, Springer, Berlin, 2013, pp. 297–305. [DOI] [PubMed]
- 93.Tao Q.F., Zeng S. Analysis of enantiomers of chiral phenethylamine drugs by capillary gas chromatography/mass spectrometry/flame-ionization detection and pre-column chiral derivatization. J. Biochem. Biophys. Methods. 2002;54:103–113. doi: 10.1016/s0165-022x(02)00134-3. [DOI] [PubMed] [Google Scholar]
- 94.Matsukawa T., Hasegawa H., Goto H. Evaluation of the metabolic chiral inversion of d-selenomethionine in rats by stable isotope dilution gas chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2015;116:59–64. doi: 10.1016/j.jpba.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 95.J. Dai, Y. Zhang, D.B. Wang-Iverson, et al., Supercritical Fluid Chromatography, John Wiley and Sons, New York, 2012, pp. 363–380.
- 96.Wang R.Q., Ong T.T., Tang W.H. Recent advances in pharmaceutical separations with supercritical fluid chromatography using chiral stationary phases. Trends Anal. Chem. 2012;37:83–100. [Google Scholar]
- 97.Liu N., Dong F., Xu J. Stereoselective determination of tebuconazole in water and zebrafish by supercritical fluid chromatography tandem mass spectrometry. J. Agric. Food Chem. 2015;63:6297–6303. doi: 10.1021/acs.jafc.5b02450. [DOI] [PubMed] [Google Scholar]
- 98.De Klerck K., Parewyck G., Mangelings D. Enantioselectivity of polysaccharide-based chiral stationary phases in supercritical fluid chromatography using methanol-containing carbon dioxide mobile phases. J. Chromatogr. A. 2012;1269:336–345. doi: 10.1016/j.chroma.2012.07.090. [DOI] [PubMed] [Google Scholar]
- 99.Wang C., Tymiak A.A., Zhang Y. Optimization and simulation of tandem column supercritical fluid chromatography separations using column back pressure as a unique parameter. Anal. Chem. 2014;86:4033–4040. doi: 10.1021/ac500530n. [DOI] [PubMed] [Google Scholar]
- 100.Belaz K.R.A., Pereira-Filho E.R., Oliveira R.V. Development of achiral and chiral 2D HPLC methods for analysis of albendazole metabolites in microsomal fractions using multivariate analysis for the in vitro metabolism. J. Chromatogr. B. 2013;932:26–33. doi: 10.1016/j.jchromb.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 101.Suntornsuk L. Recent advances of capillary electrophoresis in pharmaceutical analysis. Anal. Bioanal. Chem. 2010;398:29–52. doi: 10.1007/s00216-010-3741-5. [DOI] [PubMed] [Google Scholar]
- 102.Zhang H., Qi L., Mao L. Chiral separation using capillary electromigration techniques based on ligand exchange principle. J. Sep. Sci. 2012;35:1236–1248. doi: 10.1002/jssc.201200067. [DOI] [PubMed] [Google Scholar]
- 103.Gübitz G., Schmid M.G. Advances in chiral separation using capillary electromigration techniques. Electrophoresis. 2007;28:114–126. doi: 10.1002/elps.200600411. [DOI] [PubMed] [Google Scholar]
- 104.Hamidi S., Soltani S., Jouyban A. A dispersive liquid-liquid microextraction and chiral separation of carvedilol in human plasma using capillary electrophoresis. Bioanalysis. 2015;7:1107–1117. doi: 10.4155/bio.15.51. [DOI] [PubMed] [Google Scholar]
- 105.Staub A., Schappler J., Rudaz S. CE‐TOF/MS: fundamental concepts, instrumental considerations and applications. Electrophoresis. 2009;30:1610–1623. doi: 10.1002/elps.200800782. [DOI] [PubMed] [Google Scholar]
- 106.Ramautar R., Somsen G.W., de Jong G.J. CE‐MS for metabolomics: developments and applications in the period 2012–2014. Electrophoresis. 2015;36:212–224. doi: 10.1002/elps.201400388. [DOI] [PubMed] [Google Scholar]
- 107.Schappler J., Guillarme D., Prat J. Validation of chiral capillary electrophoresis‐electrospray ionization‐mass spectrometry methods for ecstasy and methadone in plasma. Electrophoresis. 2008;29:2193–2202. doi: 10.1002/elps.200700464. [DOI] [PubMed] [Google Scholar]
- 108.Somsen G.W., Mol R., de Jong G.J. On-line micellar electrokinetic chromatography–mass spectrometry: feasibility of direct introduction of non-volatile buffer and surfactant into the electrospray interface. J. Chromatogr. A. 2003;1000:953–961. doi: 10.1016/s0021-9673(03)00179-1. [DOI] [PubMed] [Google Scholar]
- 109.Seavey B.R., Farr E.A., Westler W.M. A relational database for sequence-specific protein NMR data. J. Biomol. NMR. 1991;1:217–236. doi: 10.1007/BF01875516. [DOI] [PubMed] [Google Scholar]
- 110.Wenzel T.J., Wilcox J.D. Chiral reagents for the determination of enantiomeric excess and absolute configuration using NMR spectroscopy. Chirality. 2003;15:256–270. doi: 10.1002/chir.10190. [DOI] [PubMed] [Google Scholar]
- 111.Płotka J.M., Biziuk M., Morrison C. Common methods for the chiral determination of amphetamine and related compounds II. Capillary electrophoresis and nuclear magnetic resonance. Trends Anal. Chem. 2012;31:23–37. [Google Scholar]
- 112.Aizawa S., Kidani T., Takada S. Simple resolution of enantiomeric NMR signals of α-amino acids by using Samarium (III) nitrate with l-tartarate. Chirality. 2015;27:353–357. doi: 10.1002/chir.22443. [DOI] [PubMed] [Google Scholar]
- 113.Pirkle W.H., Hoover D.J. NMR chiral solvating agents. Top. Stereochem. 1982;13:263–331. [Google Scholar]
- 114.Pérez-Trujillo M., Lindon J.C., Parella T. Chiral metabonomics: 1H NMR-based enantiospecific differentiation of metabolites in human urine via direct cosolvation with β-cyclodextrin. Anal. Chem. 2012;84:2868–2874. doi: 10.1021/ac203291d. [DOI] [PubMed] [Google Scholar]
- 115.Sturm S., Seger C. Liquid chromatography–nuclear magnetic resonance coupling as alternative to liquid chromatography–mass spectrometry hyphenations: curious option or powerful and complementary routine tool? J. Chromatogr. A. 2012;1259:50–61. doi: 10.1016/j.chroma.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 116.Su S., Wang Y., Bai L. Structural elucidation of in vivo metabolites of isobavachalcone in rat by LC–ESI–MSn and LC–NMR. J. Pharm. Biomed. Anal. 2015;104:38–46. doi: 10.1016/j.jpba.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 117.Wilczewska K., Kot-Wasik A., Namieśnik J. LC-MS and LC-NMR as complementary techniques for the determination of pharmaceuticals in dosage formulations. Crit. Rev. Anal. Chem. 2013;43:148–175. [Google Scholar]
- 118.Got P.A., Scherrmann J.M. Stereoselectivity of antibodies for the bioanalysis of chiral drugs. Pharm. Res. 1997;14:1516–1523. doi: 10.1023/a:1012161814494. [DOI] [PubMed] [Google Scholar]
- 119.Lequin R.M. Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA) Clin. Chem. 2005;51:2415–2418. doi: 10.1373/clinchem.2005.051532. [DOI] [PubMed] [Google Scholar]
- 120.Cao M., Li M., Yan X. Stereoselectivity of an enzyme-linked, immunosorbent assay for S-bioallethrin. Anal. Methods. 2012;4:534–538. [Google Scholar]
- 121.Izake E.L. Chiral discrimination and enantioselective analysis of drugs: an overview. J. Pharm. Sci. 2007;96:1659–1676. doi: 10.1002/jps.20820. [DOI] [PubMed] [Google Scholar]
- 122.Brocks D.R. Drug disposition in three dimensions: an update on stereoselectivity in pharmacokinetics. Biopharm. Drug Dispos. 2006;27:387–406. doi: 10.1002/bdd.517. [DOI] [PubMed] [Google Scholar]