Dear Editor:
The skin is continuously exposed to environmental insults such as microbial organisms, chemicals, and ultraviolet (UV) light. The accumulated external stimuli, especially UV radiation, induce various skin cancers including squamous cell carcinoma (SCC)1. A variety of cellular events, such as mutation of tumor suppressor p53, are implicated in the development of SCC. In addition, various intracellular molecules are involved in the regulation of SCC. Examples include S100A8 protein that regulates cutaneous SCC differentiation2. Other important contributor for SCC development and maintenance is β-catenin. Evidence shows that epidermis-specific knockout of β-catenin gene leads to the loss of cancer stem cells (or cancer initiating cells) and completes tumor regression3. It has been well established that β-catenin plays a central role in Wnt-initiated cellular signaling cascade. Once Wnt ligands bind to their cognate membrane receptors, cytoplasmic β-catenin accumulates and translocates to the nucleus, then triggering downstream gene expression. The expression of many β-catenin-regulated genes eventually affect the cellular status, which is linked to cancer development and maintenance. For example, β-catenin-regulated aldehyde dehyrognase 1A1 (ALDH1A1) is involved in gaining the stem cell characteristics of ovarian cancer4. Although the Wnt/β-catenin signaling is well recognized in various cancer cells, the downstream effectors of β-catenin in SCC are not completely identified. In this study, we attempted to identify the putative β-catenin-regulated genes in SCC.
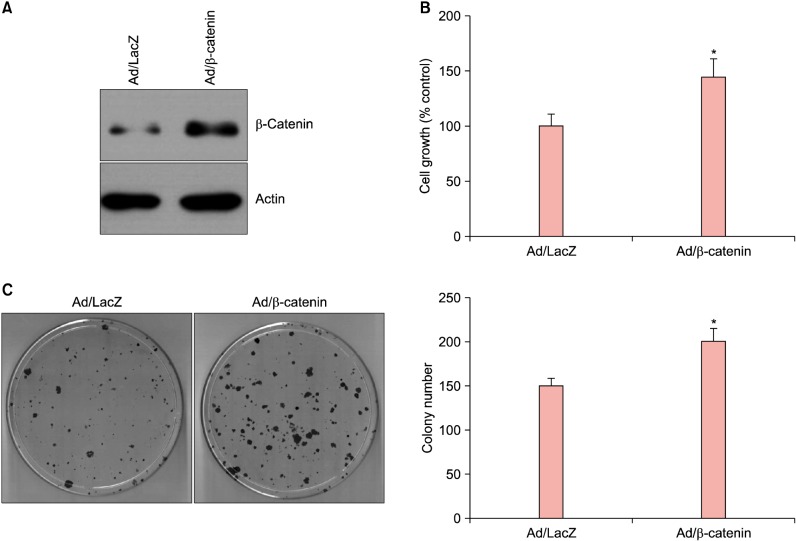
We first investigated the putative role of β-catenin in the growth and maintenance of SCC cells, using the established human squamous cell line SCC13. The SCC13 cells were cultured from SCC of the facial epidermis of 56-year-old female who received a series of radiation treatments for several years before its surgical removal5. To this end, we made a recombinant adenovirus expressing wild type β-catenin. After transduction of recombinant adenovirus into SCC13 cells, the protein level for β-catenin was highly increased compared to the control group in which adenovirus expressing LacZ was transduced (Fig. 1A). We then determined the effect of β-catenin on the growth of SCC13 cells. Overexpression of β-catenin led to the increase of cell growth compared to the control (Fig. 1B). The clonogenic assay is used for determining the cancer stem cell characteristics6. In parallel with the data on cell growth, overexpression of β-catenin markedly increased SCC13 cell colony number (Fig. 1C). These data additionally provide the evidence that β-catenin supports the growth and maintenance of SCC cells.
Fig. 1. Effects of β-catenin on the growth and maintenance of squamous cell carcinoma (SCC) cells. (A) SCC13 cells were transduced with 10 multiplicity of infections of adenovirus expressing β-catenin for overnight, then fresh growth medium was added. Cells were further incubated for 2 days, then cellular extracts were prepared and analyzed by Western blot. Adenovirus (Ad) expressing LacZ was used as a negative control. (B) SCC13 cells were transduced with a recombinant adenovirus for overnight. After replenish with fresh medium, cells were further incubated in the presence of [3H]thymidine for 2 days. Radioactivity was measured using liquid scintillation counter. The data are represented as percent control. The mean values±standard deviation are averages of triplicate measurements. *p<0.01. (C) SCC13 cells were transduced with a recombinant adenovirus for overnight. Cells were then detached from culture dish and about 2,000 cells were seeded on 100-mm dish and cultured for 2 weeks.
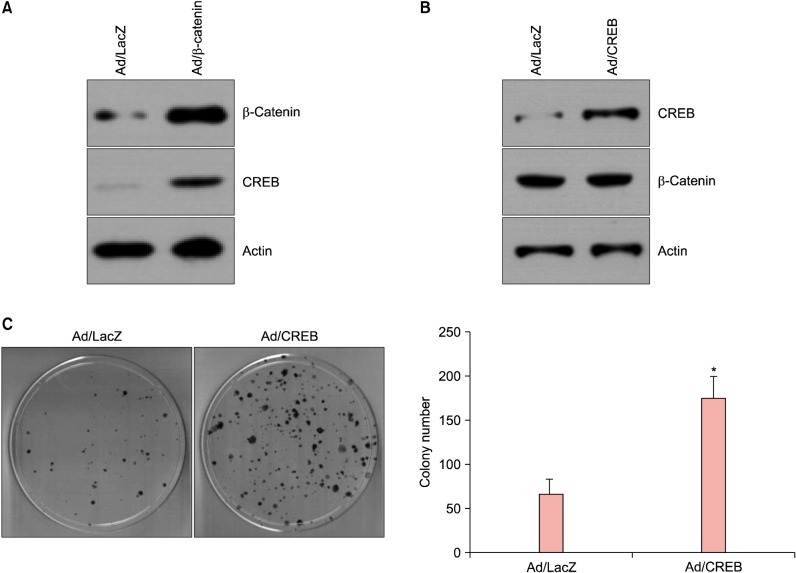
In an attempt to identify the genes regulated by β-catenin in SCC13 cells, we found that cAMP response element binding protein 1 (CREB) was induced by overexpression of β-catenin at the protein level (Fig. 2A). To investigate the potential role of CREB, we made the recombinant adenovirus expressing CREB. After adenoviral transduction into SCC13 cells, the expression of CREB was markedly increased compared to the control adenovirus-transduced cells. Overexpression of CREB, however, did not affect the protein level for β-catenin (Fig. 2B). These result suggest that CREB is a downstream molecule of β-catenin, and that there is no reciprocal regulation between β-catenin and CREB.
Fig. 2. cAMP response element binding protein 1 (CREB) as a downstream gene of β-catenin. (A) SCC13 cells were transduced with 10 multiplicity of infections (MOIs) of adenovirus expressing β-catenin for overnight, then fresh growth medium was added. Cells were further incubated for 2 days, then cellular extracts were prepared and analyzed by Western blot. CREB expression was increased in β-catenin overexpressing SCC13 cells. (B) Cells were transduced with a recombinant adenovirus expressing CREB. Overexpression of CREB did not affect the β-catenin expression. (C) SCC13 cells were transduced with a recombinant adenovirus, then cells were detached from culture dish and about 2,000 cells were seeded on 100-mm dish and cultured for 2 weeks. The mean values±standard deviation are averages of three independent experiments. *p<0.01.
To evaluate whether increased CREB affects the behavior of SCC13 cells, we performed the clonogenic assay. Overexpression of CREB resulted in marked increase of clonogenicity of SCC13 cells, more than 2-fold increase of colony formation (Fig. 2C). This data supports the role of CREB as a β-catenin downstream regulator for the growth and maintenance of SCC cells.
Multiple genomic alterations, such as mutations in tumor suppressor genes or proto-oncogenes, give rise to cancer development. In addition, many of intracellular signaling cascades are strongly implicated in cancer development and progression. Examples include sonic hedgehog and Wnt/β-catenin signaling pathways7. In this study, we investigated the putative role of β-catenin and its downstream modulator in the growth and maintenance of SCC cells. We found that β-catenin increased the proliferative potential of SCC13 cells, and that CREB was a downstream modulator of β-catenin and had also the potential for enhancing the growth and maintenance of SCC13 cells.
In solid tumors, it has been demonstrated that rare population of tumor cells in the mass can form colonies in an in vitro clonogenic assay8. These specially committed cell populations are now called the cancer stem cells and/or cancer initiating cells. Because the stem cells showed the characteristics of unlimited self-renewal, several experimental methods have been developed to study the cancer stem cells based on this characteristics. One easy method is the colony forming assay, because that stem cells are prone to grow more rapidly and make self-renewed cell populations in a colony form in culture condition9. In our study, β-catenin and its downstream CREB significantly increased the colony forming ability of SCC13 cells, suggesting that these molecules are important contributors for maintain the SCC via the regulating the cancer stem populations.
CREB is a well-known downstream effector molecule of cAMP signaling pathway. When external and/or internal stimuli increase the cytoplasmic cAMP, the intracellular protein kinase A (PKA) is activated. The activated PKA then phosphorylates the CREB, which enables the binding of CREB to its cognate DNA sequence in certain promoter of target genes10. Thus, the fact that overexpression of β-catenin led to the increase of CREB in SCC13 cells suggests that activation of β-catenin signaling can also affect intracellular signaling system such as PKA. Further study is needed to elucidate the precise crosstalk between β-catenin signaling and PKA signaling pathways in SCC development and maintenance.
In summary, we have demonstrated that β-catenin has a potential for promoting the growth and maintenance of SCC cells. Furthermore, we provide evidence that CREB is a downstream of β-catenin which is importantly involved in maintenance of SCC cells. Since β-catenin and downstream modulator CREB are heavily involved in the regulation of SCC cells, the data presented here suggest that these intracellular signaling may be the target for cure of SSC.
ACKNOWLEDGMENT
This study was supported by a grant from the National Research Foundation of Korea (NRF-2010-0023545).
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 2.Shin JM, Chang IK, Lee YH, Yeo MK, Kim JM, Sohn KC, et al. Potential role of S100A8 in cutaneous squamous cell carcinoma differentiation. Ann Dermatol. 2016;28:179–185. doi: 10.5021/ad.2016.28.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malanchi I, Peinado H, Kassen D, Hussenet T, Metzger D, Chambon P, et al. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature. 2008;452:650–653. doi: 10.1038/nature06835. [DOI] [PubMed] [Google Scholar]
- 4.Condello S, Morgan CA, Nagdas S, Cao L, Turek J, Hurley TD, et al. β-Catenin-regulated ALDH1A1 is a target in ovarian cancer spheroids. Oncogene. 2015;34:2297–2308. doi: 10.1038/onc.2014.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rheinwald JG, Beckett MA. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Cancer Res. 1981;41:1657–1663. [PubMed] [Google Scholar]
- 6.Kim HK, Moon H, Lee KS, Moon HS, Kim BS, Kim DJ. Clonogenic assay of gastric adenocarcinoma stem cells. Korean J Intern Med. 1987;2:163–169. doi: 10.3904/kjim.1987.2.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 8.Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977;197:461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- 9.Yang JS, Lavker RM, Sun TT. Upper human hair follicle contains a subpopulation of keratinocytes with superior in vitro proliferative potential. J Invest Dermatol. 1993;101:652–659. doi: 10.1111/1523-1747.ep12371671. [DOI] [PubMed] [Google Scholar]
- 10.Bossis I, Voutetakis A, Bei T, Sandrini F, Griffin KJ, Stratakis CA. Protein kinase A and its role in human neoplasia: the Carney complex paradigm. Endocr Relat Cancer. 2004;11:265–280. doi: 10.1677/erc.0.0110265. [DOI] [PubMed] [Google Scholar]