Abstract
Background
Tsutsugamushi disease is an acute, febrile, infectious disease caused by Orientia tsutsugamushi. Several studies investigating the histopathologic findings of eschars in tsutsugamushi disease reported leukocytoclastic vasculitis and neutrophil infiltration as the major findings. However, these findings may result from secondary changes following tissue necrosis. The histopathologic findings of perieschar lesions may be important to understand the primary changes associated with tsutsugamushi disease.
Objective
To investigate characteristic histopathologic features of perieschar lesions and suppose the mechanism of vascular pathophysiological changes associated with tsutsugamushi disease.
Methods
We analyzed histopathological slides of perieschar lesions in 12 patients diagnosed with tsutsugamushi disease.
Results
In the epidermis, exocytosis of mononuclear cells (75.0%) and basal vacuolar changes (66.7%) were frequent. In the dermis, perivascular, interstitial, and perineural mononuclear cell infiltration (100.0%, 83.3%, and 83.3%, respectively), as well as thrombosis (83.3%), atypical lymphocyte infiltration (91.7%), and mitotic figures (83.3%) were commonly seen. Lymphocytic vasculitis and mononuclear cell infiltration around eccrine glands were found in all cases, but eosinophil infiltration was only found in one patient (8.3%). However, the characteristic findings of eschar lesions, such as leukocytoclastic vasculitis and neutrophil infiltration, were not found in perieschar lesions.
Conclusion
The major histopathologic findings in the perieschar lesions of tsutsugamushi disease were lymphocytic vasculitis and atypical lymphocytic infiltration, mimicking lymphoma. Therefore, we suggest that this lesion should be added to the list of pseudolymphomas. To observe these characteristic histopathologic features, we also recommend that skin biopsies should be performed on perieschar lesions, not eschar lesions.
Keywords: Angiocentric lymphoma, Histopathologic finding, Lymphocytic vasculitis, Perieschar lesion, Tsutsugamushi disease
INTRODUCTION
Scrub typhus, also known as tsutsugamushi disease, is an acute, febrile, infectious disease caused by the organism Orientia tsutsugamushi. This disease, transmitted by bites of larval trombiculid mites, is endemic to the Asia-Pacific region. The clinical presentation of scrub typhus is typically characterized by fever, chills, headache, myalgia, eschar at the bite site and multiple erythematous lesions. Scrub typhus can vary in severity from mild and self-limiting to a life-threatening disease1.
Several studies have investigated the histopathologic findings of eschars characterized by a hard black crust on the skin surface in tsutsugamushi disease. Leukocytoclastic vasculitis and neutrophil infiltration were reported to be major findings in eschar lesions2. However, these findings may result from secondary changes following tissue necrosis. The histopathologic findings of perieschar lesions, which are erythematous patches or plaques around the eschar, may be important to understand primary changes associated with tsutsugamushi disease. Thus, we conducted this study to investigate characteristic histopathologic features of perieschar lesions and suppose the mechanism of vascular pathophysiological changes seen in tsutsugamushi disease.
MATERIALS AND METHODS
Patients
Twelve patients diagnosed with tsutsugamushi disease who visited Kosin University Gospel Hospital over the span of 5 years were evaluated. Diagnosis of tsutsugamushi disease was based on present illness, clinical manifestations including eschar and general symptoms, histopathologic findings, and serology results such as indirect immunofluorescent assay specific to O. tsutsugamushi.
Histopathological and immunohistochemical evaluations
A 4 mm punch biopsy was performed within 1 cm from the eschar in perieschar erythematous area. Epidermal ulcerations, parakeratosis, mononuclear cell exocytosis, individual cell necrosis, basal vacuolar changes, erythrocyte extravasation, dermal mononuclear cell infiltration (including type of infiltrated cell, infiltration site, and presence of mitotic figures), leukocytoclastic vasculitis, lymphocytic vasculitis, fibrinoid necrosis of vessel walls, thrombosis, subepidermal edema, and subcutaneous tissue panniculitis were observed in H&E-stained slides of the samples. Additionally, for tissue slices embedded in paraffin, immunohistochemical staining was performed using monoclonal antibodies for CD3, CD4, CD8, CD20, CD56, and CD68. The presence of staining for each antibody was noted, and the CD4/CD8 ratio was calculated.
RESULTS
Demographic characteristics
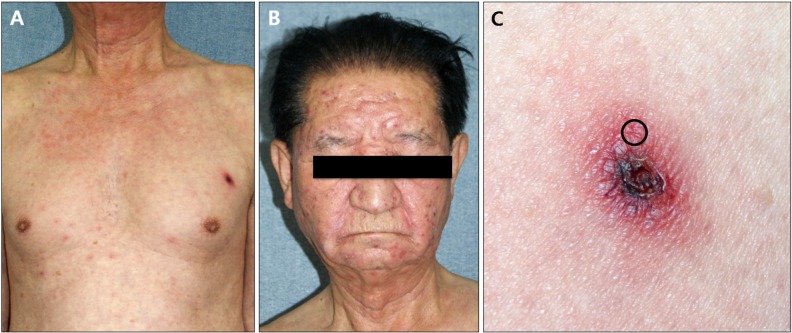
Of 12 total patients, 5 patients were males and 7 patients were females. The mean age was 50.5 years, and mean disease duration was 5.8 days. All patients had fever, while headache was observed in 8 patients; chills and myalgia were observed in 4 patients; and dizziness, nausea, and neck stiffness were observed in 1 patient each. The most frequent onset sites of eschar were the torso, legs, arms, and scalp, in that order. Laboratory findings showed increased serum glutamic oxaloacetic transaminase (S-GOT) and serum glutamic pyruvic transaminase (S-GPT) in 10 cases, increased erythrocyte sedimentation rate in 5 cases, leukocytosis in 2 cases, leukocytopenia in 1 case, hematuria in 5 cases, and proteinuria in 1 case. All patients showed positivity in the O. tsutsugamushi-specific indirect immunofluorescent assay (Fig. 1,Table 1, 2).
Fig. 1. (A) Eschar and maculopapular eruption on anterior chest. (B) Maculopapular eruption on face and neck. (C) Eschar and perieschar erythematous lesions in case 11. Skin biopsy was performed in perieschar erythematous area (circle).
Table 1. Clinical data of 12 patients with tsutsugamushi disease.
| Case | Age (yr)/sex | Location of eschar | General symptoms | Duration (d) |
|---|---|---|---|---|
| 1 | 49/female | Forearm | F | 14 |
| 2 | 52/female | Abdomen | F, H, M | 5 |
| 3 | 60/female | Axilla | F, M | 4 |
| 4 | 59/male | Abdomen, leg | C, H, F | 3 |
| 5 | 47/female | Arm | C, F, H | 7 |
| 6 | 32/male | Abdomen | F, H, N, Sn | 2 |
| 7 | 52/male | Scalp | F, H | 14 |
| 8 | 57/female | Abdomen | C, F, H, M | 7 |
| 9 | 73/female | Axilla | F | 5 |
| 10 | 32/male | Leg | C, F, H | 2 |
| 11 | 74/male | Chest | F | 4 |
| 12 | 19/Female | Leg | D, F, H, M | 3 |
F: fever, H: headache, M: myalgia, C: chill, N: nausea, Sn: neck stiffness, D: dizziness.
Table 2. Demographic data of 12 patients with tsutsugamushi disease.
| Value | |
|---|---|
| Total number of patients | 12 |
| Sex (male:female) | 5:7 |
| Mean age (yr) | 50.5 (19~74) |
| Mean duration (d) | 5.8 (2~14) |
| Location of eschar (n=13) | |
| Head and neck | 1 (7.7) |
| Trunk | 7 (53.8) |
| Arm | 2 (15.4) |
| Leg | 3 (23.1) |
| General symptoms | |
| Fever | 12 (100.0) |
| Headache | 8 (66.7) |
| Chills | 4 (33.3) |
| Myalgia | 4 (33.3) |
| Dizziness | 1 (8.3) |
| Nausea | 1 (8.3) |
| Neck stiffness | 1 (8.3) |
| Laboratory findings | |
| Leukocytosis | 2 (16.7) |
| Leukocytopenia | 1 (8.3) |
| Aspartate aminotransferase >40 IU | 10 (83.3) |
| Alanine aminotransferase >40 IU | 10 (83.3) |
| Erythrocyte sedimentation rate >20 mm/h | 5 (41.7) |
| Proteinuria | 1 (8.3) |
| Hematuria | 5 (41.7) |
| Positive reaction of immunofluorescent assay | 12 (100.0) |
Values are presented as number only, mean (range), or number (%).
Histopathological findings
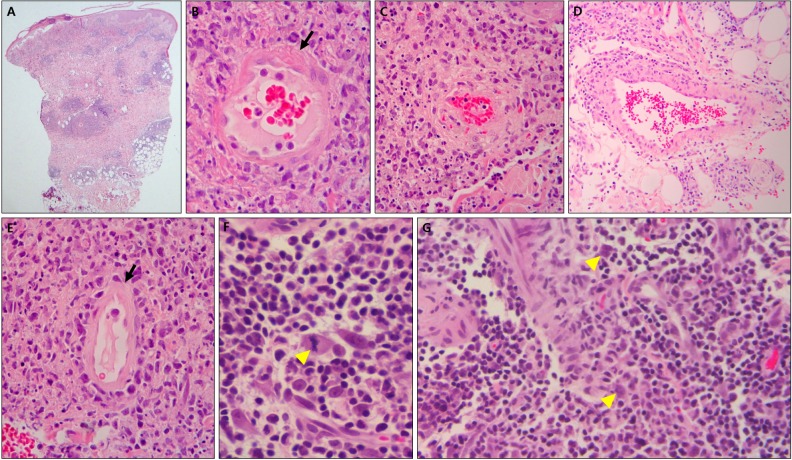
In the epidermis, parakeratosis was observed in 6 cases (50.0%), mononuclear cell exocytosis was observed in 9 cases (75.0%), individual cell necrosis was observed in 5 cases (41.7%), and basal vacuolar changes were observed in 8 cases (66.7%). Ulceration and erythrocyte extravasation were not observed in the epidermis. In the dermis, mitotic figures were observed in 10 cases (83.3%) and mononuclear cell infiltration was observed in all cases. In terms of the composition of infiltrated cells, plasma cells were observed in 11 cases (91.7%), atypical cells were observed in 11 cases (91.7%), lymphocytic dust was observed in 6 cases (50.0%), and eosinophils were observed in 1 case (8.3%). No neutrophilic infiltration was observed. In all cases, cell infiltration was observed around the blood vessels and eccrine glands. Additionally, there were 10 cases (83.3%) each of infiltration around the interstitium and nerves, and hair follicle infiltration was found in 3 cases (25.0%). Lymphocytic vasculitis, fibrinoid necrosis of the vessel wall, and erythrocyte extravasation were observed in the dermis in all cases. Thrombosis was observed in 10 cases (83.3%), and leukocytoclastic vasculitis was not observed. Ten cases (83.3%) each of subepidermal edema and subcutaneous tissue panniculitis were observed (Fig. 2, Table 3).
Fig. 2. (A) Histopathologic findings show dense infiltration around vessels and eccrine glands in dermis and subcutaneous tissue (H&E, ×40). (B, C) Lymphohistiocytic infiltration around vessels, fibrinoid necrosis (arrow) of vessel wall, thrombus deposition and halo within vessel, and lymphoid dust are shown (H&E, ×400). (D) Vessel wall with hyalinization is rimmed by abundant lymphohistiocytes (H&E, ×200). (E) Vessel lumen with attenuated endothelium (arrow) contains numerous red blood cells (H&E, ×400). (F) Mitotic figure (arrowhead; H&E, ×1,000) and (G) large atypical pleomorphic cells with hyperchromatic nuclei (arrowheads) are shown in dermis (H&E, ×400) (case 5).
Table 3. Histopathologic findings of cutaneous lesions in 12 specimens from perieschar erythematous lesions of tsutsugamushi disease (n=12).
| Histopathologic feature | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Patient (n, %) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermis | |||||||||||||
| Ulceration | − | − | − | − | − | − | − | − | − | − | − | − | 0 (0) |
| Parakeratosis | − | − | − | − | + | − | + | + | + | + | + | − | 6 (50.0) |
| Exocytosis of mononuclear cells | − | − | + | + | + | + | + | + | − | + | + | + | 9 (75.0) |
| Individual cell necrosis | + | − | + | − | − | + | − | − | + | + | − | − | 5 (41.7) |
| Basal vacuolar changes | − | + | + | − | − | + | + | + | + | − | − | − | 8 (66.7) |
| Erythrocyte extravasation | − | − | − | − | − | − | − | − | − | − | − | − | 0 (0) |
| Dermis | |||||||||||||
| Mononuclear cell infiltration | |||||||||||||
| Perivascular | + | + | + | + | + | + | + | + | + | + | + | + | 12 (100.0) |
| Interstitial | + | + | − | + | + | + | + | − | + | + | + | + | 10 (83.3) |
| Perineural | + | + | − | + | + | − | + | + | + | + | + | + | 10 (83.3) |
| Perifollicular | − | − | − | − | − | + | + | − | − | + | − | − | 3 (25.0) |
| Around eccrine glands | + | + | + | + | + | + | + | + | + | + | + | + | 12 (100.0) |
| With neutrophils | − | − | − | − | − | − | − | − | − | − | − | − | 0 (0) |
| With eosinophils | − | − | − | − | − | − | − | + | − | − | − | − | 1 (8.3) |
| With plasma cells | + | + | + | − | + | + | + | + | + | + | + | + | 11 (91.7) |
| With atypical cells | + | + | − | + | + | + | + | + | + | + | + | + | 11 (91.7) |
| With mitotic figures | + | − | − | + | + | + | + | + | + | + | + | + | 10 (83.3) |
| With lymphocytic dusts | − | + | − | − | + | + | − | + | − | − | + | + | 6 (50.0) |
| Vasculitis | |||||||||||||
| Leukocytoclastic | − | − | − | − | − | − | − | − | − | − | − | − | 0 (0) |
| Lymphocytic | + | + | + | + | + | + | + | + | + | + | + | + | 12 (100.0) |
| Fibrinoid necrosis of vessel walls | + | + | + | + | + | + | + | + | + | + | + | + | 12 (100.0) |
| Extravasation of erythrocytes | + | + | + | + | + | + | + | + | + | + | + | + | 12 (100.0) |
| Thrombosis | + | − | − | + | + | + | + | + | + | + | + | + | 10 83.3) |
| Subepidermal edema | + | − | + | + | + | + | − | + | + | + | + | + | 10 (83.3) |
| Subcutaneous tissue | |||||||||||||
| Panniculitis | + | − | + | + | + | + | + | + | − | + | + | + | 10 (83.3) |
Immunohistochemical findings
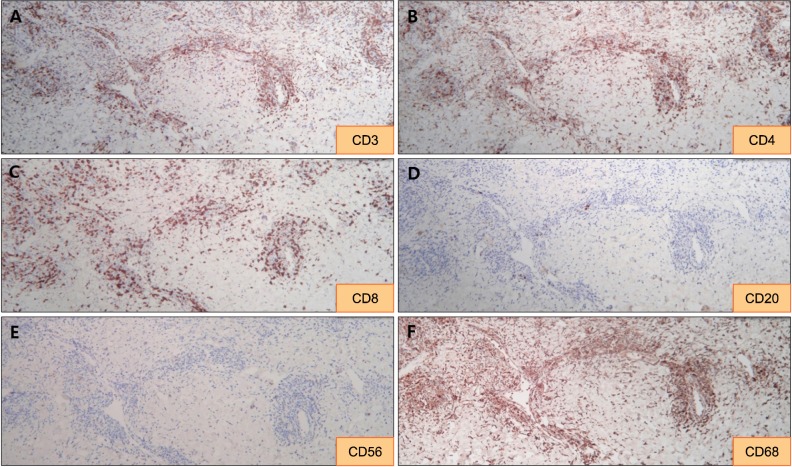
In each of the three cases for which immunohistochemical staining was performed, CD3 and CD68 were strongly positive. Also, CD4-positive T cells were more common than CD8-positive T cells. Finally, CD20 and CD56 were negative in each case (Fig. 3, Table 4).
Fig. 3. (A~C) Immunohistochemically, the majority of infiltrating atypical lymphocytes are CD3-positive T-cells with a predominance of CD4-positive cells over CD8-positive cells (×100). (D~F) Perivascular and interstitial infiltrating cells are negative for CD20 and CD56 and positive for CD68 (case 11) (×100).
Table 4. Immunohistochemical findings of cutaneous lesions in 3 specimens from perieschar erythematous lesions of tsutsugamushi disease.
| Case | Immunophenotype | ||||
|---|---|---|---|---|---|
| CD3 | CD4/CD8 ratio | CD20 | CD56 | CD68 | |
| 6 | +++ | >1 | − | − | +++ |
| 9 | +++ | >1 | − | − | +++ |
| 11 | +++ | >1 | − | − | +++ |
−: <1%, +: 1%~25%, ++: 25%~50%, +++: >50% of cells.
DISCUSSION
Scrub typhus, also known as tsutsugamushi disease, is an infectious disease caused by O. tsutsugamushi, a parasite within the cells of mites. Scrub typhus is passed through bites of trombiculid mites, which belong to the Trombiculidae family. Following bites from trombiculid mites, an eschar and a systemic, asymptomatic rash appear. In most cases, the eschar does not have subjective symptoms, and it varies in frequency from 55% to 82%. The most common areas of eschar onset include the armpit, buttocks, narrow parts, and sites typically covered by underwear. Anemia, leukocytosis, leukocytopenia, thrombocytopenia, and hypoalbuminemia are present in some cases. A South Korean study reported that tsutsugamushi disease was accompanied by increased S-GOT and S-GPT in approximately 80% of cases3.
Allen and Spitz4 reported that in biopsies of eschar areas, acanthosis, cellular swelling, and necrosis were found in the epidermis. Compact infiltration of monocytes consisting of lymphocytes, plasma cells, mast cells, histiocytes, and Reed-Sternberg cells was observed around sweat glands, hair follicles, sebaceous glands, nerves, and blood vessels in the dermis. They also noted that collagen denaturation similar to coagulative changes accompanying other insect bites are sometimes present along with vasodilation, acute thrombophlebitis, and arteritis. Chaudhary et al.2 reported that in biopsies of the eschar areas, keratinocyte necrosis, basal vacuolar degeneration, and red blood cell (RBC) entrapment were observed in the epidermis in approximately 20% of cases. In the dermis, leukocytoclastic vasculitis, neutrophil infiltration, and RBC extravasation were observed in 93.3%, 86.7%, and 93.3% of cases, respectively, but lymphocytic vasculitis was observed only in 6.7% of the cases. In addition, vascular wall necrosis, swelling of vascular endothelial cells and thrombosis have reportedly been observed. Vasculitis in tsutsugamushi disease is currently thought to be caused by either destruction of endothelial cells in small vessels or an inflammatory response caused by leukocytes5. In this study, the histopathological findings of lesions around the eschar included lymphocytic vasculitis and infiltration of atypical lymphohistiocytes around blood vessels, sweat glands, hair follicles, sebaceous glands, and nerves. Leukocytoclastic vasculitis and neutrophil infiltration were not present, but atypical cell infiltration, mitotic figures, vascular wall necrosis, and thrombosis were observed in this study.
In leukocytoclastic vasculitis, circulating immune complexes are deposited on the vascular wall, the complement system is activated, and various inflammatory mediators are isolated to destroy endothelial cells6. Hypercoagulability was found in 53% of patients with an ulcer caused by leukocytoclastic vasculitis7. In addition, in the case of ulcers caused by a local injury, leukocytoclastic vasculitis is often found on histopathological examination. For such cases of vasculitis, it is important to consider the possibility of secondary phenomena8. Some authors define lymphocytic vasculitis as a condition involving lymphocyte infiltration around blood vessels and fibrinoid necrosis of the vascular wall. In this scenario, lymphocytes cause direct damage to vascular endothelial cells and induce changes to the vascular wall, such as vascular fibrin deposition, coagulation, and vascular endothelial growth9. In psoriasis and granulomatous vasculitis, lymphocyte-medicated vasculitis develops in early stages of the disease. The resulting immune complexes cause an inflammatory response involving leukocytes. The same report also highlighted the possibility of leukocyte infiltration and leukocytoclasia, which are secondarily seen in many diseases after vascular damage due to lymphocytes10. Lymphocytes can also activate other inflammatory cells, including leukocytes, and can play an important role in leukocytoclastic vasculitis by activating Langerhans cells11. The reports to date on leukocytoclastic vasculitis in tsutsugamushi disease indicate that studies on the histopathological findings of lesions around eschars are not sufficient. When erythematous papules caused by bites of larval trombiculid mites progress to ulcers or eschars, coagulative necrosis develops at a histopathological level, primarily due to lymphocytic vasculitis and thrombosis. As a secondary response to this, circulating immune complexes are deposited on the vascular wall and leukocytoclastic vasculitis accompanied by keratinocyte necrosis and neutrophil infiltration in the epidermis of the eschar area appear. Therefore, the vasculitis of tsutsugamushi disease is thought to be lymphocytic vasculitis and is not caused by leukocytes. To observe this phenomenon, lesions around eschars should be biopsied, not the eschar area itself.
Like tsutsugamushi disease, insect bites, NK/T cell lymphoma, and lymphomatoid granulomatosis can present with lymphocytic vasculitis. These diseases differ from tsutsugamushi disease in treatment and prognosis, and differentiation is very important. In the case of insect bites, clinical symptoms vary depending on the causative insect. In general, insect bites appear as erythematous macules or papules and blisters. On a histopathological level, edema of the papillary dermis and infiltration of lymphocytes and eosinophils around blood vessels within the reticular dermis are commonly seen. Eosinophil infiltration is rare in tsutsugamushi disease6, and it was not observed in most cases (91.7%) of this study. Thus, eosinophil infiltration is helpful in differentiating between insect bites and tsutsugamushi disease. NK/T cell lymphoma consists of either Epstein-Barr-virus (EBV)-positive NK cells or, rarely, of T cells showing cytotoxicity. Indurated plaques or tumors may develop, and ulcers are common. These ulcers may show similar clinical manifestations to eschars found in tsutsugamushi disease. Histopathologically, lymphocytes, histiocytes, plasma cells, Reed-Sternberg cells, and atypical lymphocytes infiltrate the dermal and subcutaneous fat layers. Mitotic figures are often observed, angiocentric infiltration is noticeable, blood vessel destruction is severe, and necrosis of the surrounding tissues occurs. Most cases of NK/T cell lymphoma are CD56 positive, EBV positive, TIA-1 positive, granzyme positive, perforin positive, and CD3 negative. Lymphomatoid granulomatosis primarily invades the lungs, and skin symptoms such as erythematous macules, papules, plaques, and nodules appear in approximately 40% of cases12. It is classified as a B cell lymphoma, and its pathogenesis involves EBV. Histopathologically, it is characterized by vascular destruction and vascular infiltration of lymphocytes and histiocytes. Atypical lymphocytes and mitotic figures are also observed, and it is CD20, CD3, and CD4 positive13. Tsutsugamushi disease involves infiltration of atypical lymphohistiocytes (consisting of lymphocytes, histiocytes, plasma cells, Reed-Sternberg cells, and atypical lymphoid cells), which can also be observed in angiocentric lymphoma. Tsutsugamushi disease also involves mitotic figures and vascular invasion, and the disease is included in the category of pseudolymphoma. It is important to differentiate between these diseases by completely understanding their clinical, histopathological, and immunohistochemistry findings.
In this study, the clinical manifestations and laboratory findings of tsutsugamushi disease were investigated. By observing the histopathological findings of lesions around eschars, we confirmed that tsutsugamushi disease is considered a pseudolymphoma. The major findings were lymphocytic vasculitis and infiltration of atypical lymphohistiocytes around blood vessels, mimicking angiocentric lymphoma. Especially, leukocytoclastic vasculitis and neutrophil infiltration were not observed in all cases. This is significant because the findings are different from histopathological findings of the eschar areas that have been reported to date. This result indicates that the existing proposed mechanism of vasculitis in tsutsugamushi disease should be adjusted. In conclusion, the vasculitis of tsutsugamushi disease is not caused by neutrophils but is instead a lymphocytic vasculitis. To observe this finding, biopsies should be performed on lesions around the eschar, instead of on the eschar area itself.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Ogawa M, Hagiwara T, Kishimoto T, Shiga S, Yoshida Y, Furuya Y, et al. Scrub typhus in Japan: epidemiology and clinical features of cases reported in 1998. Am J Trop Med Hyg. 2002;67:162–165. doi: 10.4269/ajtmh.2002.67.162. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhary N, Lee JS, Wu JY, Tharin S. Evidence for use of teriparatide in spinal fusion surgery in osteoporotic patients. World Neurosurg. 2017;100:551–556. doi: 10.1016/j.wneu.2016.11.135. [DOI] [PubMed] [Google Scholar]
- 3.Hu ML, Liu JW, Wu KL, Lu SN, Chiou SS, Kuo CH, et al. Short report: abnormal liver function in scrub typhus. Am J Trop Med Hyg. 2005;73:667–668. [PubMed] [Google Scholar]
- 4.Allen AC, Spitz S. A comparative study of the pathology of scrub typhus (tsutsugamushi disease) and other rickettsial diseases. Am J Pathol. 1945;21:603–681. [PMC free article] [PubMed] [Google Scholar]
- 5.Seong SY, Choi MS, Kim IS. Orientia tsutsugamushi infection: overview and immune responses. Microbes Infect. 2001;3:11–21. doi: 10.1016/s1286-4579(00)01352-6. [DOI] [PubMed] [Google Scholar]
- 6.Soter NA. Cutaneous necrotizing venulitis. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, editors. Fitzpatrick's dermatology in general medicine. 8th ed. New York: McGraw Hill; 2012. pp. 2003–2008. [Google Scholar]
- 7.Mekkes JR, Loots MA, van der Wal AC, Bos JD. Increased incidence of hypercoagulability in patients with leg ulcers caused by leukocytoclastic vasculitis. J Am Acad Dermatol. 2004;50:104–107. doi: 10.1016/s0190-9622(03)00881-8. [DOI] [PubMed] [Google Scholar]
- 8.Barksdale SK, Scumpia P, Wang JY, Xu X, Barnhill RL. Vascular disease. In: Elder DE, Elinitsas R, Rosenbach M, Murphy GF, Rubin AI, Xu X, editors. Lever's histopathology of the skin. 11th ed. Philadelphia: Lippincott William & Wilkins; 2015. pp. 240–275. [Google Scholar]
- 9.Massa MC, Su WP. Lymphocytic vasculitis: is it a specific clinicopathologic entity? J Cutan Pathol. 1984;11:132–139. doi: 10.1111/j.1600-0560.1984.tb00363.x. [DOI] [PubMed] [Google Scholar]
- 10.Kossard S. Defining lymphocytic vasculitis. Australas J Dermatol. 2000;41:149–155. doi: 10.1046/j.1440-0960.2000.00419.x. [DOI] [PubMed] [Google Scholar]
- 11.Paris DH, Phetsouvanh R, Tanganuchitcharnchai A, Jones M, Jenjaroen K, Vongsouvath M, et al. Orientia tsutsugamushi in human scrub typhus eschars shows tropism for dendritic cells and monocytes rather than endothelium. PLoS Negl Trop Dis. 2012;6:e1466. doi: 10.1371/journal.pntd.0001466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chi DH, Huh JR, Sung KJ, Koh JK. Epstein-Barr virus associated cutaneous angiocetric immunoproliferative lesion showing histologic features of classical lymphomatoid granulomatosis. Ann Dermatol. 1996;8:110–113. [Google Scholar]
- 13.Cerroni L. Other cutaneous B-cell lymphomas. In: Cerroni L, editor. Skin lymphoma: the illustrated guide. 4th ed. Oxford: Wiley-Blackwell; 2014. pp. 253–256. [Google Scholar]