Abstract
Background
While cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) is the most commonly used chemotherapeutic regimen for patients with peripheral T-cell lymphomas (PTCLs), elderly patients are more vulnerable to associated toxicities. We evaluated the efficacy and safety of dose-attenuated CHOP in elderly patients with PTCL.
Methods
Patients with PTCL aged >70 years or 65–70-years with comorbidities were treated with dose-attenuated CHOP (cyclophosphamide: 562.5 mg/m2, doxorubicin: 37.5 mg/m2, vincristine: 1.4 mg/m2, and prednisolone: 100 mg for five days; 25% reduced dose of cyclophosphamide and doxorubicin vs. full-dose CHOP) as first-line therapy were included.
Results
Forty-four patients (median age, 74 yr) were analyzed. The majority (N=42, 95.5%) had advanced stage disease and 36 (81.8%) were classified as high/high-intermediate risk by the international prognostic index. The overall response rate was 61.4%, and 21 patients achieved complete response (47.7%). With median follow-up period of 28.8 months, the estimated two-year progression-free and overall survival rates were 36.7% and 46.6%, respectively. Grade 3/4 neutropenia and thrombocytopenia occurred in 26.9% and 7.4% of 204 total cycles, which affected 76.7% and 25.6% of the patients, respectively. Nineteen patients (44.2%) experienced febrile neutropenia, and six died due to treatment-related toxicities. High lactate dehydrogenase levels and an involvement of >1 extranodal sites were prognostic indicators of poor survival.
Conclusion
Dose-attenuated CHOP does not compromise treatment efficacy but retains significant toxicity. Our results suggest that some patients can be effectively treated with dose-attenuated CHOP, however a novel therapy for elderly patients with PTCL is required.
Keywords: Peripheral T cell lymphoma, Elderly, Dose-attenuated CHOP
INTRODUCTION
Peripheral T cell lymphoma (PTCL) represents a group of heterogeneous lymphoproliferative disorders arising from the clonal expansion of post-thymic T cells or natural killer (NK) cells [1]. While PTCL is a relatively uncommon disease, which accounts for <20% of all non-Hodgkin lymphoma (NHL) cases, the prevalence of PTCL in Asian countries is higher than in the West [2,3]. The clinical course of PTCLs is typically aggressive and associated with poor prognosis with five-year overall survival (OS) rates below 30% [3,4,5,6].
The median age for PTCL development was reported to be 62 years in the international T-cell lymphoma projects, and according to SEER data, approximately 40% of patients with PTCL are above the age of 70 years [3,7]. Moreover, elderly patients have a poor tolerance to chemotherapy due to impaired bone marrow (BM) function, altered drug metabolism, and presence of comorbid diseases. Thus, elderly patient with PTCL often suffer from increased susceptibility to treatment-related complications [6,8].
Although there is no consensus based on randomized trials regarding an optimal first-line chemotherapy for PTCLs, anthracycline-based CHOP-like regimens have been the most common treatment. However, optimal dosing for elderly patients has not been specifically addressed. In this study, we attempted to reduce the dose of two myelotoxic agents (cyclophosphamide and doxorubicin) by 25% from the full-dose CHOP regimen, with the aim of decreasing chemotherapy-related hematologic and/or infectious complications in elderly patients. We assessed the efficacy and safety of a dose-attenuated CHOP regimens in patients with PTCL aged over 70 years and those aged between 65–70-year with comorbidities that were either expected to result in significant treatment-related toxicities or included in the parameters of a comprehensive geriatric assessment.
MATERIALS AND METHODS
Patient population
The data of 44 consecutive elderly patients with PTCL were used for analysis in this study. We included patients aged between 65 and 70 years, with comorbidities that were expected to result in significant treatment-related toxicities or included in the parameters of a comprehensive geriatric assessment [such as heart failure (NYHA III-IV), coronary artery disease, cardiac arrhythmia, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, renal insufficiency (creatinine clearance ≤50 mL/min), cirrhosis, post-stroke neurologic sequelae, and/or medically unfit condition] [9,10], and patients aged over 70 years who were treated with dose-attenuated CHOP chemotherapy between April 2001 and 2015. All patients underwent pre-treatment heart scan or transthoracic echocardiography, and those with significant cardiac dysfunction were excluded due to risk of anthracycline cardiotoxicity. Diagnosis of PTCL was confirmed by a certified hematopathologist, according to World Health Organization (WHO) criteria [11]. We excluded patients with extranodal NK/T cell lymphoma, nasal-type (ENKTL) or adult T cell leukemia/lymphoma, and those who had previously received other chemotherapeutic agents for lymphoma. The study protocol was approved by the Institutional Review Board (2016-0568) in accordance with the 2008 Declaration of Helsinki.
Disease evaluation
All patients were staged according to the Lugano classification at the time of diagnosis based on results of their medical history, physical examination, complete blood count, serum lactate dehydrogenase (LDH), computed tomography (CT), BM aspiration and biopsy, and a positron emission tomography-CT (PET-CT) scan [12]. Response to treatment was assessed after four and six cycles of dose-attenuated CHOP chemotherapy or whenever progressive disease was suspected based on international criteria [13]. Hematologic and major treatment-related adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
Treatment
The dose-attenuated CHOP regimen consisted of 562.5 mg/m2 cyclophosphamide administered intravenously for one day (25% reduction from full-dose CHOP), 37.5 mg/m2 doxorubicin for one day (25% reduction from full-dose CHOP), 1.4 mg/m2 vincristine for one day, and 50 mg prednisolone twice a day for five days. Patients were scheduled to receive six cycles of dose-attenuated CHOP at three-week intervals, however the treatment interval could be modified at the physician's discretion after considering blood count recovery or the patients' general condition. Primary prophylactic pegylated granulocyte-colony stimulating factor (peg-G-CSF) was routinely administered to nine patients treated since August 2014, when peg-G-CSF was reimbursed by national health insurance. In the event of severe neutropenia or febrile neutropenic events, G-CSF was administered subcutaneously to patients who were not given prophylactic peg-G-CSF. The dose of cyclophosphamide and doxorubicin was reduced by an additional 25% if the ANC and platelet counts did not recover to less than grade 1, even after a two-week delay, or according to physician discretion.
Statistical analysis
Overall survival (OS) was calculated from the date of the diagnosis to the date of last follow-up or death due to any cause. Progression-free survival (PFS) was defined as the duration from the date of diagnosis to the date of progression, relapse, or death from any cause. Efficacy analyses were performed using the intention-to-treat principle. Survival was calculated using the Kaplan–Meier method and analyzed using a log-rank test for univariate analysis and a Cox regression model for multivariate analysis. Variables with P<0.1 in the univariate analysis were selected for the multivariate analysis models. Two-sided P<0.05 was considered statistically significant.
RESULTS
Patient characteristics
Characteristics of the 44 elderly patients with PTCL are summarized in Table 1. The diagnoses included PTCL-not otherwise specified (NOS) (N=27), angioimmunoblastic T cell lymphoma (N=9), anaplastic lymphoma kinase (ALK)-negative anaplastic large cell lymphoma (N=5), and enteropathy-associated T cell lymphoma (N=3). The median age at diagnosis was 74 years (range, 65–86 yr). Of the 15 patients aged between 65 and 70 years, there were three with diabetes mellitus and hypertension, one with diabetes mellitus and angina, three who had an immobilized status associated with a compression fracture, one with arrhythmia (atrial fibrillation), one with liver cirrhosis, one with post-stroke neurologic sequelae, one with myelodysplastic syndrome, one with major depression, one with chronic lung disease, one with poor nutritional status with post-op leakage after small bowel resection, and one with panhypopituitarism. Three patients were identified as positive for hepatitis B surface antigen before treatment, two of whom were already on treatment with entecavir and the other one was given prophylactic tenofovir with chemotherapy. Most patients displayed an advanced disease stage (95.5%), and 36 (81.8%) patients were categorized as high-intermediate or high risk according to the International Prognostic Index (IPI) scoring system [14]. According to the Prognostic Index for PTCL-U (PIT), which includes age, Eastern Cooperative Oncology Group performance status (ECOG PS), LDH level, and BM involvement, 79.5% of the patients were classified into a high-risk group (PIT score>2) [4].
Table 1. Patient characteristics.
Abbreviations: AITL, angioimmunoblastic T cell lymphoma; ALCL, anaplastic large cell lymphoma; ALK, anaplastic lymphoma kinase; EATL, enteropathy-associated T-cell lymphoma; ECOG, Eastern Cooperative Oncology Group; IPI, international prognostic index; LDH, lactate dehydrogenase; PIT, Prognostic Index for PTCL-U; PTCL-NOS, peripheral T-cell lymphoma-not otherwise specified; WHO, World Health Organization.
Response to treatment
Of the 44 patients in the intention-to-treat population, 23 (52.3%) patients completed the six scheduled cycles of treatment. The causes of discontinuation included chemo-refractory or progressive disease within three cycles (N=11), infection (N=6), subdural hemorrhage (N=1), and intolerance (N=2), and one patient was lost to follow-up. Relative dose intensity for the 23 patients who completed treatment was 72.4±13.7% for both doxorubicin and cyclophosphamide. The response to treatment was assessable in 35 patients both during and at the end of treatment, however nine patients died or were lost to follow-up before the post-treatment evaluation. A total of 21 patients achieved complete response (CR) and six achieved partial response (PR); thus, the overall response rate (ORR) and CR rate were 61.4% and 47.7%, respectively. Of the 21 complete responders, 11 remained in remission for a median duration of 14.3 months (range, 1.4–158.1 mo), however 9 of the 21 complete responders and four of the six partial responders experienced disease relapse or progression after completing treatment. Of the other two patients who achieved PR, one died of pneumonia with septic shock 12 days after achieving PR, following three cycles of the chemotherapy, and the other was lost to follow-up four months after completion of six chemotherapy cycles.
Survival
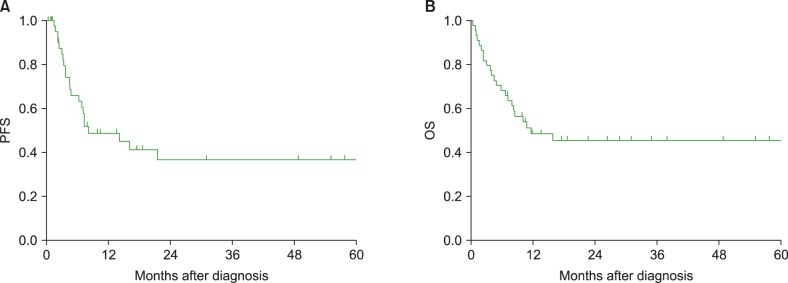
During the median follow-up duration for survivors (28.8 mo, range, 7.1–174.6 mo), 23 patients experienced disease progression and 23 patients died. The estimated two-year PFS and OS rates were 36.7% (95% CI: 27.9–45.5%) and 46.6% (95% CI: 37.7–53.3%), and the median PFS and OS were 8.2 and 11.7 months, respectively (Fig. 1). Of the 23 deaths, 16 were secondary to lymphoma progression, six were related to treatment toxicities (five infections and one bleeding), and one was due to an unknown cause.
Fig. 1. Survival curves. (A) The estimated two-year progression-free survival rate was 36.7% and (B) the two-year overall survival rate was 46.6%.
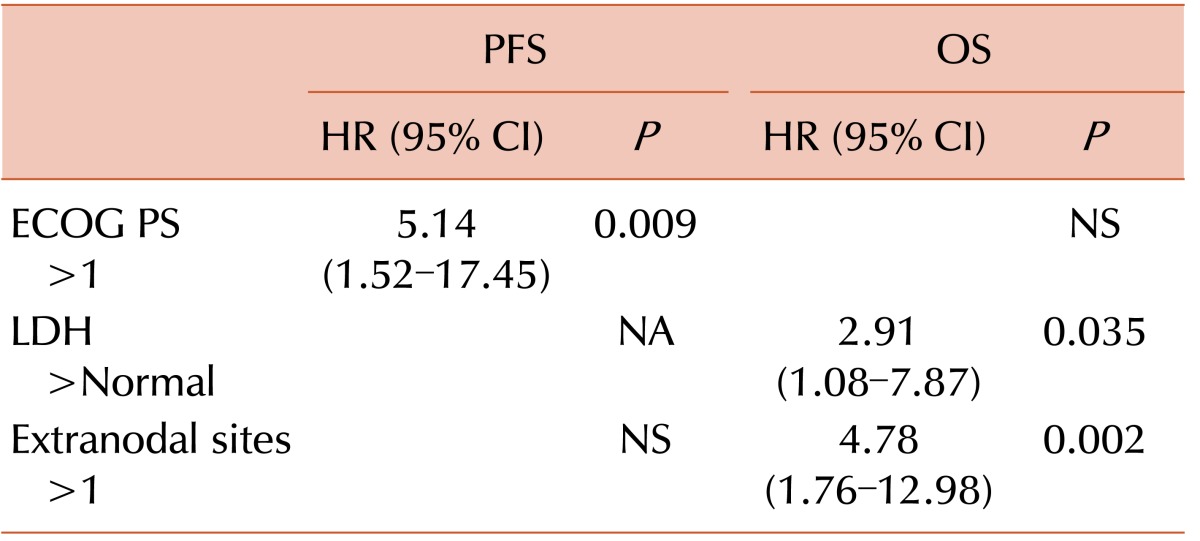
Of the 27 responders, 20 remained alive at the time of the analysis, whereas only one of eight non-responders survived. The surviving patient who did not respond to dose-attenuated CHOP received four cycles of gemcitabine-based cytotoxic chemotherapy (gemcitabine, dexamethasone, and cisplatin) as salvage treatment, achieved CR, and remained alive at the time of the analysis without evidence of disease. Thirteen patients survived for more than 48 months at the time of analysis, and these long-term survivors showed tendencies toward better performance status (ECOG PS 2–4 of 7.7% vs. 30.2%, P=0.1), less advanced stage (Ann Arbor stage III-IV of 76.9% vs. 100%, P=0.001), and less extranodal disease (>1 extranodal sites of 38.5% vs. 62.8%, P=0.120) than short-term survivors at baseline. In addition, long-term survivors had higher CR rates (92.3% vs. 39.5%, P=0.004) and experienced less grade 3 or 4 thrombocytopenia (0% vs. 35%, P=0.013) and neutropenia (61.5% vs. 82.5%, P=0.117). In the univariate analysis, ECOG PS >1 (P=0.023), high IPI score (P=0.043), and high PIT score (P=0.028) were associated with inferior PFS (Table 2). Patients with ECOG PS >1 (P=0.019), high LDH level (P=0.013), or disease involvement of >1 extranodal sites (P=0.003) had a shorter OS duration (Table 2). The Cox-regression model analysis revealed that poor ECOG PS [hazard ratio (HR): 5.14; 95% CI: 1.52–17.45; P=0.009] remained an independent prognostic factor for shorter PFS. The involvement of more than one extranodal site (HR: 4.78; 95% CI: 1.76–12.98; P=0.002) and a high LDH level (HR: 2.91; 95% CI: 1.08–7.87; P=0.035) were independent prognostic factors of inferior OS (Table 3).
Table 2. Univariate analysis of prognostic factors.
Abbreviations: AITL, angioimmunoblastic T cell lymphoma; ALCL, anaplastic large cell lymphoma; ALK, anaplastic lymphoma kinase; BM, bone marrow; EATL, enteropathy-associated T-cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; IPI, international prognostic index; LDH, lactate dehydrogenase; OS, overall survival; PFS, progression-free survival; PIT, Prognostic Index for PTCL-U; PTCL-NOS, peripheral T-cell lymphoma-not otherwise specified; WHO, World Health Organization.
Table 3. Multivariate analysis of prognostic factors.
Abbreviations: CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; LDH, lactate dehydrogenase; NA, not applicable; NS, not significant; OS, overall survival; PFS, progression-free survival.
Treatment toxicity
Hematologic toxicities were evaluable in 43 of the 44 patients; one patient was lost to follow-up after the first cycle of chemotherapy. Grade 3 or 4 neutropenia was observed after 55 (26.9%) of the 204 total administered cycles, which affected 33 (76.7%) of 43 patients. Of these 55 neutropenic events, 42 (76.4%) occurred within the second cycle of chemotherapy. A total of 19 (44.2%) patients experienced febrile neutropenia during treatment, and three died of infectious complications. Grade 3 or 4 thrombocytopenia occurred after 15 (7.4%) out of 204 total cycles, which affected 11 (25.6%) of 43 patients. Although the difference was not significant, patients with BM involvement at the time of diagnosis experienced more severe neutropenia after chemotherapy, which was denoted by nadir ANC median counts of 345/µL with BM involvement, and 601/µL without BM involvement (P=0.074). Further dose reduction was necessary for 16 of 44 patients (36.4%) due to severe neutropenia that resulted in febrile neutropenic episodes. Among the nine patients who received primary prophylactic peg-G-CSF, seven (77.8%) and three (33.3%) patients experienced grade 3 or 4 neutropenia and febrile neutropenia, respectively. Of the 35 patients who did not receive prophylactic peg-G-CSF, 28 (80.0%) and 16 (45.7%) experienced grade 3 or 4 neutropenia and febrile neutropenia, respectively (P=0.754 for grade 3 or 4 neutropenia; P=0.461 for febrile neutropenia).
The most common non-hematologic serious adverse events were infections, and five patients (11.4%) experienced grade 3 or 4 infectious complications. Among these five patients, four died from septic shock within 28 days after the first cycle of chemotherapy. Five patients (11.4%) experienced grade 2 or higher sensory neuropathy during treatment, and one suffered from persistent cytopenia and a subdural hemorrhage, six weeks after the second cycle.
DISCUSSION
In our study, we evaluated the outcomes of dose-attenuated CHOP chemotherapy for elderly PTCLs, which resulted in an ORR of 61.4%, CR rate of 47.7%, two-year PFS rate of 36.7%, and OS of 46.6%. These results appear comparable to those of previous studies involving full-dose CHOP chemotherapy that covered a 21-day interval. In the NHL-B2 trial of the DSHNHL, patient with B- and T-cell NHL aged between 61 and 75 years exhibited a CR rate of 60.1%, three-year event-free survival of 41.3%, and three-year OS of 48.8% [15]. In the GOELAMs-LTP95 prospective randomized study of PTCL, which compared VIP-reinforced ABVD with CHOP-21, 69% of patients were aged over 60 years, and the CR and two-year PFS rates in the CHOP group were 35% and 41%, respectively [16]. Recent multicenter data from South East Korea assessing the efficacy of various regimens, such as CHOP, CHOEP, IMEP, and CVP, in elderly patients with PTCL also reported an ORR of 70.2% with a CR rate of 37.8%, and the estimated five-year PFS and OS rates were 16.6% and 45.9%, respectively, which are similar to our results [17].
In the present study, a dose reduction of doxorubicin and cyclophosphamide failed to mitigate the toxicities associated with CHOP chemotherapy in elderly patients. The reduced treatment dose was still associated with elevated rates of hematologic toxicities, including grade 3 or 4 neutropenia and febrile neutropenic events in 76.7% and 44.2% of patients, respectively. This high rate of neutropenia was similar to the rate of 72.1% exhibited in the three-weekly CHOP arm of the NHL-B2 trial of DSHNHL [15]. In the GOELAMS-LTP95 trial, grade 3/4 neutropenia and thrombocytopenia episodes were observed in 8.3% and 1.6% of all cycles, respectively, with treatment-related deaths occurring in 6.7% of patients [16]. These might be attributed to reduced hematopoietic reserve, decreased hepatic and renal function associated with drug metabolism and excretion, changes in physiological body composition related to increased toxicity, and emotional frailty of elderly patients [18]. In the present study, 19 (43.2%) of the 44 patients had lymphoma with BM involvement at the time of diagnosis, and this incidence was much higher than the 3% to 29% rates of common PTCL subtypes obtained from an international PTCL study [3]. A Chinese study involving 56 patients with PTCL patients who were over 60 years in age also reported a substantially smaller proportion of BM involvement, at 7.0% [19]. Our results suggest that this high rate of BM involvement at least partially explains the higher frequencies of grade 3 or 4 neutropenia and febrile neutropenia events observed in our study. Old age is generally considered a risk factor for higher rates of neutropenic infections following CHOP treatment or CHOP-like regimens [20,21], and several controlled trials and meta-analyses have revealed that the prophylactic use of peg-G-CSF reduces the occurrence of febrile neutropenic events [22,23]. Although the present study included nine patients who received primary prophylactic peg-G-CSF, no significant differences in grade 3 or 4 neutropenia and febrile neutropenia were noted during the routine administration of prophylactic G-CSF, which could be attributed to the small number of patients in our study. In addition, six patients died of therapy-related toxicities without disease progression, which yielded a slightly higher treatment-related mortality rates (13.6%) than observed in the two previous studies: 7% in the GOELAMS-LTP95 trial and 3.4% in the three weekly CHOP arm of the NHL-B2 trial of DSHNHL. However, this difference requires cautious interpretation because patients from the NHL-B2 trial of DSHNHL had far better baseline patient characteristics than that of our study cohort, including substantially lower rates of BM involvement (12.9%), advanced stage (53.4%), IPI-high risk (25.3%), and extranodal sites >1 (30.9%).
The major limitations of our study include its retrospective nature and small sample size. Our approach did not include a steroid prephase, which is advocated by the German high-grade lymphoma study group and might increase patient tolerability to chemotherapy [18]. Despite these limitations, our results address a very important question pertaining to the proper dose of CHOP chemotherapy for frail elderly patients. It is especially noteworthy that the regimen did not compromise efficacy despite the dose reduction, which suggests that at least some patients can be treated with reduced dose CHOP without compromising the efficacy of this therapy. We believe that full-dose chemotherapy is not mandatory in frail elderly populations, as the outcomes of intensive chemotherapies were not more favorable than those of CHOP therapy in the MDACC study [24] or a separate randomized trial [16]. In theory, through effectively separating these two populations of patients, we may be able to discover trends towards better performance status, early stage disease, and less involvement of extranodal disease in long-term survivors.
In conclusion, the efficacy of dose-attenuated CHOP appears comparable to that of full-dose CHOP chemotherapy in elderly patients. The short duration of response with frequent relapses and a high toxicity rates remain serious shortcomings associated with this regimen. However, we identified a subgroup of long-term survivors with durable responses and strategies are required to effectively identify such patients. In addition, safer and more effective novel therapeutic approaches for elderly populations are urgently needed.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Savage KJ, Harris NL, Vose JM, et al. ALK− anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111:5496–5504. doi: 10.1182/blood-2008-01-134270. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non-Hodgkin's lymphomas: distributions of the major subtypes differ by geographic locations. Non-Hodgkin's Lymphoma Classification Project. Ann Oncol. 1998;9:717–720. doi: 10.1023/a:1008265532487. [DOI] [PubMed] [Google Scholar]
- 3.Vose J, Armitage J, Weisenburger D International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 4.Gallamini A, Stelitano C, Calvi R, et al. Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study. Blood. 2004;103:2474–2479. doi: 10.1182/blood-2003-09-3080. [DOI] [PubMed] [Google Scholar]
- 5.Weisenburger DD, Savage KJ, Harris NL, et al. Peripheral T-cell lymphoma, not otherwise specified: a report of 340 cases from the International Peripheral T-cell Lymphoma Project. Blood. 2011;117:3402–3408. doi: 10.1182/blood-2010-09-310342. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz N, Trümper L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418–3425. doi: 10.1182/blood-2010-02-270785. [DOI] [PubMed] [Google Scholar]
- 7.Adams SV, Newcomb PA, Shustov AR. Racial patterns of peripheral T-cell lymphoma incidence and survival in the United States. J Clin Oncol. 2016;34:963–971. doi: 10.1200/JCO.2015.63.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2011;12:460–468. doi: 10.1016/S1470-2045(11)70069-9. [DOI] [PubMed] [Google Scholar]
- 9.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25:1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Older Adult Oncology. Fort Washington, PA: National Comprehensive Cancer Network; 2016. [Accessed April 11, 2017]. at https://www.nccn.org/professionals/physician_gls/pdf/senior.pdf. [Google Scholar]
- 11.Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC Press; 2008. [Google Scholar]
- 12.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 14.International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 15.Pfreundschuh M, Trümper L, Kloess M, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood. 2004;104:634–641. doi: 10.1182/blood-2003-06-2095. [DOI] [PubMed] [Google Scholar]
- 16.Simon A, Peoch M, Casassus P, et al. Upfront VIP-reinforced-ABVD (VIP-rABVD) is not superior to CHOP/21 in newly diagnosed peripheral T cell lymphoma. Results of the randomized phase III trial GOELAMS-LTP95. Br J Haematol. 2010;151:159–166. doi: 10.1111/j.1365-2141.2010.08329.x. [DOI] [PubMed] [Google Scholar]
- 17.Jo JC, Choi Y, Shin HJ, et al. Peripheral T cell lymphomas in elderly patients: a retrospective analysis from the Hematology Association of South East Korea (HASEK) Ann Hematol. 2016;95:619–624. doi: 10.1007/s00277-016-2597-y. [DOI] [PubMed] [Google Scholar]
- 18.Pfreundschuh M. How I treat elderly patients with diffuse large B-cell lymphoma. Blood. 2010;116:5103–5110. doi: 10.1182/blood-2010-07-259333. [DOI] [PubMed] [Google Scholar]
- 19.Zhao H, Wang T, Wang Y, et al. Comorbidity as an independent prognostic factor in elderly patients with peripheral T-cell lymphoma. Onco Targets Ther. 2016;9:1795–1799. doi: 10.2147/OTT.S93687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bastion Y, Blay JY, Divine M, et al. Elderly patients with aggressive non-Hodgkin's lymphoma: disease presentation, response to treatment, and survival-a Groupe d'Etude des Lymphomes de l'Adulte study on 453 patients older than 69 years. J Clin Oncol. 1997;15:2945–2953. doi: 10.1200/JCO.1997.15.8.2945. [DOI] [PubMed] [Google Scholar]
- 21.Bertini M, Freilone R, Vitolo U, et al. The treatment of elderly patients with aggressive non-Hodgkin's lymphomas: feasibility and efficacy of an intensive multidrug regimen. Leuk Lymphoma. 1996;22:483–493. doi: 10.3109/10428199609054787. [DOI] [PubMed] [Google Scholar]
- 22.Grigg A, Solal-Celigny P, Hoskin P, et al. Open-label, randomized study of pegfilgrastim vs. daily filgrastim as an adjunct to chemotherapy in elderly patients with non-Hodgkin's lymphoma. Leuk Lymphoma. 2003;44:1503–1508. doi: 10.1080/1042819031000103953. [DOI] [PubMed] [Google Scholar]
- 23.Lyman GH, Kuderer N, Agboola O, Balducci L. Evidence-based use of colony-stimulating factors in elderly cancer patients. Cancer Control. 2003;10:487–499. doi: 10.1177/107327480301000607. [DOI] [PubMed] [Google Scholar]
- 24.Escalón MP, Liu NS, Yang Y, et al. Prognostic factors and treatment of patients with T-cell non-Hodgkin lymphoma: the M.D. Anderson Cancer Center experience. Cancer. 2005;103:2091–2098. doi: 10.1002/cncr.20999. [DOI] [PubMed] [Google Scholar]