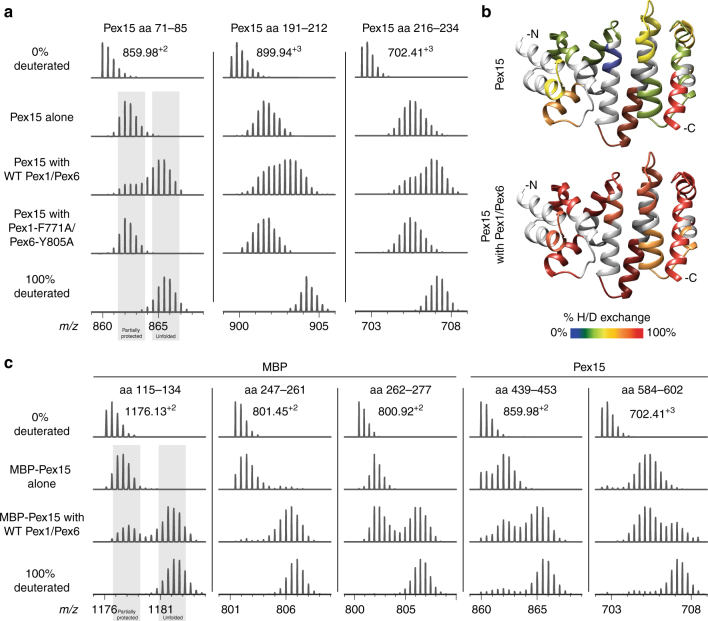
Fig. 3.
Pex1/Pex6 unfolding of Pex15 measured by HDX-MS. a Mass spectra of selected Pex15 peptides after a 60 s pre-incubation alone, with wild-type Pex1/Pex6, or with pore-loop mutant Pex1/Pex6 followed by 15 s of deuteration. Control spectra of no deuteration and 100% deuteration are shown for comparison. b The crystal structure of the Pex15 core domain is colored according to the relative deuteration levels of Pex15 peptides after a 30 s deuteration in isolation (top) or in the presence of wild-type Pex1/Pex6 motor (bottom). Regions without peptide coverage are colored light gray. c Mass spectra of selected peptides from an MBP-Pex15 fusion demonstrate that Pex1/Pex6 can unfold both MBP and Pex15 moieties. For each peptide, spectra are shown for MBP-Pex15 after 60 s incubation alone or in the presence of Pex1/Pex6, followed by a 15 s deuteration