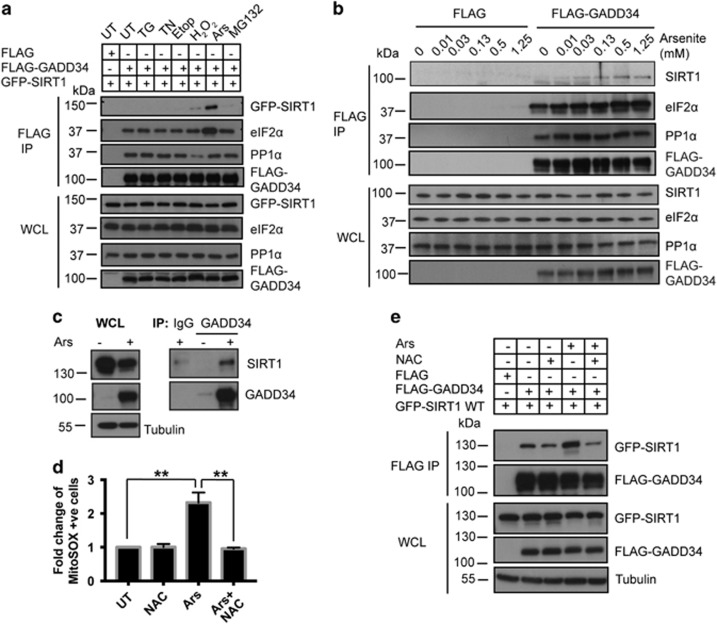
Figure 2.
Ars enhances SIRT1 association with GADD34. (a) HEK293 cells expressing FLAG-GADD34 were subjected to the following stresses for 1 h (hr): TG, TN, Etop, H2O2, Ars and MG132 before immunoprecipitations (IP) using anti-FLAG antibody. IP and WCL were immunoblotted for GFP-SIRT1, FLAG-GADD34, eIF2α and PP1α. Molecular weight markers (kDa) are shown. (b) HEK293 cells expressing the FLAG peptide or FLAG-GADD34 were subjected to increasing Ars concentrations for 1 h before IP using anti-FLAG. IP and WCL were immunoblotted for SIRT1, eIF2α, PP1α and FLAG-GADD34. (c) Anti-GADD34 immunoprecipitates from WT MEFs treated with Ars (50 μM, 5 h) or UT were immunoblotted for SIRT1 and GADD34. Control IgG antibody was included for control IP. Tubulin was used as loading control. (d) HEK293 cells, exposed to antioxidant, NAC (20 mM, 2 h), or Ars (0.5 mM, 1 h) or NAC (20 mM, 1 h) before Ars (0.5 mM, 1 h), were stained using mitochondrial ROS-detecting dye, MitoSOX. MitoSOX-stained cells were quantified by FACS and represented as fold change compared with UT HEK293 (mean±S.E.M., n=3). Sidak’s multiple comparisons test was used after two-way ANOVA to generate P-values (**P<0.01). (e) Anti-FLAG immunoprecipitations from HEK293 cells co-expressing FLAG-GADD34 and GFP-SIRT1 WT either UT (−) or following treatment with NAC, Ars or NAC and Ars as described in d were analyzed. IP and WCL were immunoblotted for GFP-SIRT1 and FLAG-GADD34. Tubulin was used as a loading control. Molecular weight markers (kDa) are shown