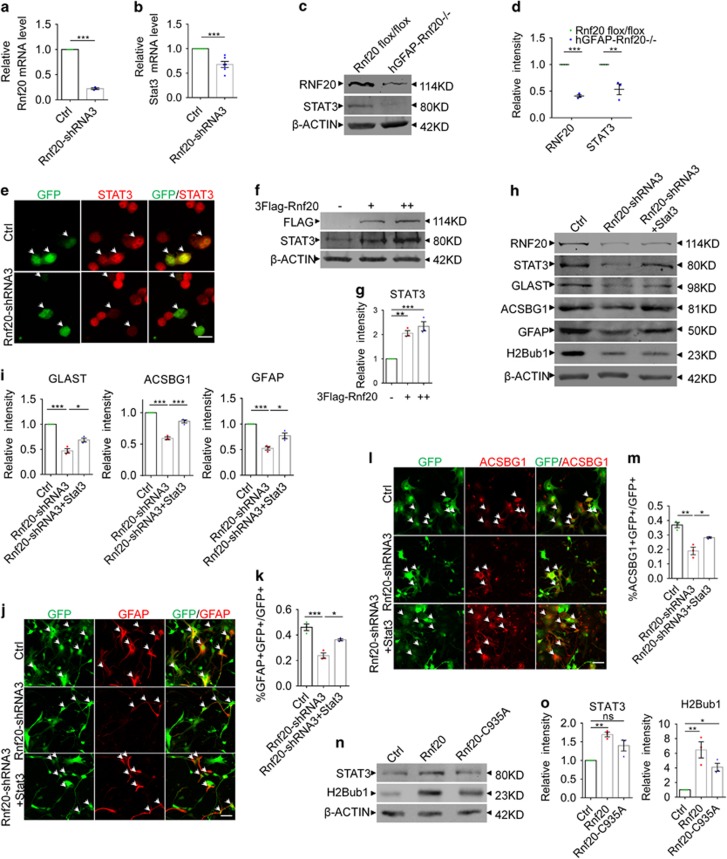
Figure 6.
RNF20 promotes the expression of STAT3. (a and b) Real-time PCR showed that RNF20 knockdown in E16 neural precursor cells reduced the relative mRNA level of STAT3 under differentiation condition for 4 days. Mean±S.E.M.; n=3 for Rnf20, n=6 for Stat3. (c) Western blotting showed the reduction of RNF20 and STAT3 in dorsal cortices of hGFAP-Rnf 20−/− mice at P0. (d) Protein levels of RNF20 and STAT3 in (c) were quantified. Mean±S.E.M.; n=3. (e) N2a cells were transfected with control or Rnf20-shRNA3 plasmids, and cultured for 48–72 h. Immunostaining showed Rnf20-shRNA3 reduces the immunoreactivity of STAT3. Scale bar, 20 μm. (f) Western blotting showed the increase of STAT3 expression is dependent on the dosage of RNF20. N2a cells were transfected with control or 3Flag-Rnf20 plasmids and cultured for 48–72 h. +, 1 μg; ++, 2 μg. (g) Protein levels of RNF20 and STAT3 in (f) were quantified. Mean±S.E.M.; n=3. (h) E16 neural precursor cells were infected with control or Rnf20-shRNA3 lentivirus, or co-infected with Rnf20-shRNA3 and Stat3 lentivirus, and cultured for 4 days under differentiation condition. Western blotting showed STAT3 can rescue the astrocyte production deficiency. RNF20, STAT3, GLAST, ACSBG1, GFAP and H2Bub1 were detected with indicated antibodies. (i) Protein levels of GLAST, ACSBG1 and GFAP in (h) were quantified. Mean±S.E.M.; n=3. (j and l) Immunostaining of GFAP and ACSBG1 showed that overexpression of STAT3 partially rescued the deficiency caused by Rnf20 knockdown. E16 neural precursor cells were isolated, infected and differentiated for 4 days. Scale bar, 50 μm. (k and m) Percentage of GFAP- or ACSBG1-positive cells was quantified. Mean±S.E.M.; n=3. (n) The mutant Rnf20-C935A failed to increase the expression of STAT3. N2a cells were transfected with control, Rnf20 or Rnf20-C935A plasmids, and cultured for 48–72 h. (o) Protein levels of STAT3 in (n) were quantified. Mean±S.E.M.; n=3. *P<0.05, **P<0.01, ***P<0.001, Student’s t-test for (a, b and d), one-way ANOVA for (g, i, k, m and o)