Abstract
Introduction
We examined the impact of autoantibodies on the erythropoietin receptor (EPOR) in type 2 diabetic patients with chronic kidney disease (CKD).
Methods
A total of 112 Japanese patients with type 2 diabetes who had CKD were enrolled in this study and followed for a mean of 45 months. Sera from these patients were screened for anti-EPOR antibodies using enzyme-linked immunosorbent assays.
Results
Anti-EPOR antibodies were detected in 26 patients (23%). Anti-EPOR antibodies were associated with low hemoglobin concentrations and decreased renal function. In patients with biopsy-proven diabetic nephropathy, anti-EPOR antibodies were associated with increased levels of interstitial inflammation. A decrease in renal function was observed more frequently in patients with antibodies than in those without antibodies, and the presence of the antibodies together with well-known clinical parameters, including proteinuria and low glomerular filtration rate, was a significant risk factor for end-stage renal disease. In human tubular epithelial HK-2 cells, IgG fractions containing anti-EPOR antibodies upregulated the expression of monocyte chemoattractant protein-1 mRNA under a high concentration of glucose.
Conclusion
Anti-EPOR antibodies might be involved in the progression of renal lesions and in the impaired erythropoiesis in type 2 diabetic patients with CKD. Furthermore, the presence of anti-EPOR antibodies may be an additional predictor for end-stage renal disease in type 2 diabetes.
Keywords: autoantibodies, diabetic nephropathy, erythropoietin receptor, prognosis, risk factor
Diabetes and its complications are major causes of morbidity and mortality in most countries.1 Among diabetic complications, nephropathy occurs in 20% to 40% of patients during the course of their disease.2 Although kidney disease attributable to diabetes is referred to as diabetic nephropathy or diabetic kidney disease, diabetes and various kidney diseases have become common chronic conditions.3 Thus, the prevalence of chronic kidney disease (CKD) is increasing in proportion to the increase in diabetes, and it has been predicted to continue to increase in the future.4 Diabetes is a risk factor for cardiovascular disease and death, and CKD increases this risk further.5, 6, 7, 8 Anemia is commonly observed in diabetic patients with CKD and is involved in the increased risk for the progression of kidney disease and cardiovascular mortality and morbidity.7, 9, 10, 11
Anemia in diabetic patients with CKD may result from ≥1 mechanisms. Among these, the major causes are iron and erythropoietin (EPO) deficiencies, as well as hyporesponsiveness to the action of EPO.11 In view of EPO hyporesponsive anemia, we previously detected and reported autoantibodies to the EPO receptor (EPOR) as a possible cause of anemia with erythroid hypoplasia.12 In that study, these antibodies were unexpectedly detected even in diabetic patients with CKD, although their clinical significance remains to be investigated.12 In addition, a recent study revealed that anti-EPOR antibodies were associated with overall disease activity and the decline of renal function in patients with systemic lupus erythematosus.13
These results prompted us to examine the clinical and pathological impact of anti-EPOR antibodies in type 2 diabetic patients with CKD. We found that anti-EPOR antibodies were detected in a subset of patients, were associated with renal lesions, and that they were inversely related to the preservation of renal function.
Materials and Methods
Patients
A total of 112 type 2 diabetic patients who had been diagnosed with CKD and followed at Kanazawa University Hospital between 1989 and 2014 were included in this study. The mean follow-up was 45.3 ± 47.4 months. Patients with secondary diabetes, renal transplantation, or dialysis were excluded. Among the enrolled patients, 51 underwent a renal biopsy. A diagnosis of diabetic nephropathy was confirmed by histological characteristics using renal biopsy specimens, including light microscopy, electron microscopy, and immunofluorescence examination. A renal biopsy was performed for the precise diagnosis of renal lesions with the consent of each patient. All blood samples were obtained after the patients gave their written informed consent at admission for the renal biopsy, or for workup and treatment of the disease. The study protocol adhered to the Declaration of Helsinki and was approved by the medical ethics committee of Kanazawa University. In addition to 40 previously reported healthy control subjects,12 a further 8 healthy individuals were included as control subjects in the present study, and as a result, anti-EPOR antibodies were not detected in serum samples from any of these subjects.
Clinical Features and Routine Laboratory Tests
Demographic and clinical features were evaluated for each enrolled patient. Baseline clinical and laboratory findings, including the use of angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and erythropoiesis-stimulating agents, were extracted from medical records. Twenty-four hour urinary protein excretion, serum creatinine, estimated glomerular filtration rate (eGFR), glycosylated hemoglobin (HbA1c), body mass index, systolic and diastolic blood pressures, total cholesterol, hemoglobin (Hb), iron, total iron binding capacity, ferritin, and C-reactive protein were used as baseline clinical parameters at admission. eGFR for Japanese patients was calculated using the following equation: eGFR (ml/min per 1.73 m2) = 194 × serum creatinine−1.094 × age−0.287 (if female, × 0.739).14 HbA1c levels were presented as National Glycohemoglobin Standardization Program values according to the recommendations of the Japan Diabetic Society15 and the International Federation of Clinical Chemistry values. Autoantibodies against glutamic acid decarboxylase were measured by a commercial quantitative enzyme-linked immunosorbent assay (SRL, Inc., Tokyo, Japan).
Detection of Autoantibodies to EPOR
Anti-EPOR antibodies were detected by enzyme-linked immunosorbent assay as described previously.12 Briefly, polyvinyl 96-well microtitration plates (Nunc International, Tokyo, Japan) were coated with recombinant human EPOR (R & D Systems, Minneapolis, Minnesota) at 5 μg/ml diluted in 0.2 M sodium bicarbonate buffer at 4°C for 24 hours. The remaining free-binding sites were blocked with 1% bovine serum albumin in phosphate-buffered saline at 4°C. After the plates were washed with Tween 20-Tris-buffered saline, the samples were added in duplicate at 1:1000 dilution to 1% bovine serum albumin in phosphate-buffered saline for 20 hours at 4°C. The plates were washed 4 times with the same buffer and incubated with goat antihuman Ig-conjugated with horseradish peroxidase (Millipore, Temecula, California) at 1:5000 dilution for 1.5 hours at room temperature. The substrate tetramethylbenzidine (KPL, Gaithersburg, Maryland) was added, and the reaction was stopped by the addition of 2 N sulfuric acid. The optical density at 450 nm (OD450) was determined by an automatic plate reader, and the sample was considered to be antibody-positive when the ratio of patient serum OD450 to that of normal control sera was ≥2.4. This cutoff OD value was determined by a preliminary analysis using a receiver-operating characteristic curve to predict, sensitively and specifically, renal outcome among the enrolled type 2 diabetic patients with a positive OD value ≥1.5 (data not shown).12
Purification of IgG Fractions
Sera from patients and control subjects were clarified by centrifugation at 1500g for 20 min (500 μl) and filtration through 0.45-μm filters (Millipore, County Cork, Ireland). IgG fractions were prepared using a MAb Trap Kit (GE Healthcare, Tokyo, Japan) according to the manufacturer’s instructions. Purified IgG fractions were concentrated using Centriprep centrifugal filters (Millipore) and stored at 4°C until required.
Histopathological Studies
Renal biopsy specimens were prepared for light microscopic examination as described previously.7 In brief, the samples of the patients were fixed in 10% phosphate-buffered formalin (pH 7.2), embedded in paraffin, and sliced into 4-μm sections. These specimens were stained with hematoxylin and eosin, periodic acid Schiff (PAS) reagent, Mallory-Azan, and periodic acid silver methenamine, and were examined under a light microscope. The severity of diffuse lesions of glomeruli was graded on a scale of 0 to 4 as described previously: grade 0, all glomeruli appear normal; grade 1, local lesions present within each glomerulus, and focal lesions present within the kidney; grade 2, mesangial thickening is diffuse within the glomerulus and generalized throughout the kidney; grade 3, capillary lumina are narrowed and obliterated only locally; and grade 4, the lumen is generally narrowed, and the entire glomerulus is ischemic and appears to be hyalinized.7 Nodular lesions, exudative lesions, and mesangiolysis were simply shown by their presence or absence in each specimen.7 The severity of interstitial fibrosis and tubular atrophy (IFTA) and interstitial inflammation was scored according to the criteria of the Renal Pathology Society.16 The severity of IFTA was evaluated and graded on a scale from 0 to 3: grade 0, no IFTA; grade 1, <25%; grade 2, 25% to 50%; and grade 3, >50%.16 The severity of interstitial inflammation was evaluated and graded on a scale from 0 to 2: grade 0, absent; grade 1, infiltration only in relation to IFTA; and grade 2, infiltration in areas without IFTA.16 The severity of arteriolar hyalinosis was evaluated and graded on a scale from 0 to 3 as described previously: grade 0, normal appearance without PAS-positive deposits; grade 1, a light PAS-positive thickening is observed but at less than one-half the circumference of the arteriole in many arterioles; grade 2, most vessel walls are moderately thickened with PAS-positive deposition without apparent luminal narrowing; and grade 3, a heavy thickening of most vessel walls is seen with luminal narrowing or obliteration.7 The severity of arteriosclerosis was evaluated and graded on a scale from 0 to 2 according to the Renal Pathology Society criteria: grade 0, no intimal thickening; grade 1, intimal thickening less than the thickness of the media; and grade 2, intimal thickening greater than the thickness of the media.16 Renal tissue specimens were examined by 4 nephrologists.
Renal Outcome
The outcome for this study was end-stage renal disease (ESRD), which was defined as the need for dialysis or renal transplantation, as described elsewhere.
Cells and Cell Culture
The human renal tubular epithelial HK-2 cell line was grown according to the manufacturer’s instructions (CRL-2190; ATCC, Manassas, Virginia). To examine the effect of purified IgG fractions with or without anti-EPOR antibodies on the function of HK-2 cells under diabetic conditions, HK-2 cells were incubated with regulated concentrations of D-glucose (Wako Chemicals, Inc., Tokyo, Japan) and/or EPO. Briefly, HK-2 cells (3.0 × 105/ml) were cultured in Dulbecco’s modified Eagle’s medium supplemented with 0.5% heat-inactivated fetal bovine serum (Gibco BRL Life Technologies, Carlsbad, California) at 37°C in a humidified atmosphere with 5% carbon dioxide for 24 hours after isolation in the presence of 5 or 20 mmol/l of D-glucose. Furthermore, IgG fractions with or without anti-EPOR antibodies from patients were applied. HK-2 cells (1.5 × 105/ml) were preincubated with IgG (10 μg) in each culture medium for 60 min. Subsequently, HK-2 cells were incubated in the presence of recombinant human EPO (10 ng/ml) (Thermo Fisher Scientific, Inc., Yokohama, Kanagawa, Japan) for 48 hours.
Reverse Transcription-Polymerase Chain Reaction
The transcript of human EPOR was detected using the reverse transcription-polymerase chain reaction (RT-PCR). In brief, total RNA was extracted from cultured HK-2 cells under a normal or high concentration of glucose, and complementary DNA (cDNA) was reverse-transcribed from 1 μg total RNA using an RT-PCR kit (Takara Shuzo, Tokyo, Japan). The cDNA product was amplified by PCR as follows: incubation for 3 min at 94°C, followed by 35 cycles of 1 min at 94°C, 2 min at 55°C, and 3 min at 72°C, and a final extension for 7 min at 72°C.17 Primers for human EPOR (sense, 5′-GCA-CCG-AGT-GTG-TGC-TGA-CGA-A-3′; antisense, 5′-GGT-CAG-CAG-CAC-CAG-CAT-GAC-3′) were used to examine its expression.18 The human EPO-dependent cell line AS-E212, 19 was used as a positive control. The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase was used as a PCR control.20
Real-Time Quantitative PCR
To determine transcripts of monocyte chemoattractant protein-1 (MCP-1), total RNA was extracted from cultured HK-2 cells as described previously. Quantitative real-time RT-PCR was performed on the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, California). Assay identifications of TaqMan gene expression assays were Hs00234140 for MCP-1 and Hs99999905 for glyceraldehyde-3-phosphate dehydrogenase.
Statistical Analysis
All comparisons between the 2 patient groups were performed using the χ2 test and Mann-Whitney U test. A renal outcome curve was obtained using the Kaplan-Meier method and compared by using the log-rank test. A multivariate Cox proportional hazards regression model was used to select factors that significantly affected the incidence of renal outcome and to estimate risk. The following variables were incorporated as covariates: age, sex, body mass index, systolic blood pressure, proteinuria, eGFR, HbA1c, Hb, and the presence of anti-EPOR antibodies. A multiple comparison among 3 groups in in vitro experiments was performed using Tukey’s test. All analyses were carried out using the statistical package SPSS (version 22; SPSS, Tokyo, Japan) and Stata 14.2 software (Stata Corporation, College Station, Texas). P values <0.05 were considered statistically significant.
Results
Characteristics of the Enrolled Patients
None of the 112 patients enrolled had antiglutamic acid decarboxylase antibodies. The baseline characteristics of the 112 patients are shown in Table 1. The mean age of the patients was 62.9 years, and 76 (67.9%) were men. The mean amount of urinary protein excretion was 2.7 g/d. The mean serum creatinine concentration was 2.4 mg/dl, and the mean eGFR was 42.0 ml/min per 1.73 m2. The median Hb concentration and reticulocyte count were 11.2 g/dl and 4.4 × 104/ml, respectively. Diabetes-related parameters included a mean duration of 13.6 years at the time of workup on admission and a mean HbA1c of 6.7%. The mean levels of serum iron, total iron binding capacity, and ferritin were 71 mg/dl, 244 mg/dl, and 233 ng/ml, respectively. The mean C-reactive protein level was 0.6 mg/dl.
Table 1.
Baseline clinical parameters for all patients and those with and without anti- erythropoietin receptor (EPOR) antibodies
| Clinical parameters | All patients (n = 112) | Anti-EPOR negative (n = 86) | Anti-EPOR positive (n = 26) | P value |
|---|---|---|---|---|
| Male | 76 | 58 | 18 | 0.86 |
| Age (yr) | 62.9 ± 12.5 | 61.5 ± 12.3 | 67.5 ± 12.2 | <0.05 |
| Proteinuria (g/d) | 2.7 ± 3.1 | 2.7 ± 3.1 | 2.8 ± 3.1 | 0.9 |
| Serum creatinine (mg/dl) | 2.4 ± 2.2 | 2.1 ± 2.0 | 3.1 ± 2.4 | <0.05 |
| eGFR (ml/min per1.73 m2) | 42.0 ± 30.2 | 45.4 ± 30.2 | 30.7 ± 27.7 | <0.05 |
| Duration of diabetes (yr) | 13.6 ± 9.8 | 13.1 ± 9.9 | 15.0 ± 9.7 | 0.41 |
| HbA1c (%) | 6.7 ± 1.5 | 6.8 ± 1.6 | 6.4 ± 1.3 | 0.22 |
| BMI (kg/m2) | 24.3 ± 4.0 | 24.7 ± 4.1 | 22.6 ± 3.3 | <0.05 |
| SBP (mm Hg) | 136 ± 24 | 135 ± 23 | 137 ± 27 | 0.75 |
| DBP (mm Hg) | 74 ± 14 | 75 ± 14 | 72 ± 14 | 0.42 |
| T-Chol (mg/dl) | 180 ± 51 | 186 ± 50 | 160 ± 47 | <0.05 |
| HDL-C (mg/dl) | 45 ± 18 | 47 ± 20 | 40 ± 12 | 0.12 |
| Triglyceride (mg/dl) | 142 ± 79 | 152 ± 85 | 108 ± 39 | <0.01 |
| Hemoglobin (g/dl) | 11.2 ± 2.0 | 11.5 ± 2.0 | 10.1 ± 1.7 | <0.01 |
| Reticulocytes (×104/μl)a | 4.4 ± 2.2 | 4.4 ± 2.0 | 4.3 ± 2.7 | 0.96 |
| Fe (mg/dl) | 71.2 ± 40.6 | 73.3 ± 43.2 | 66.3 ± 34.0 | 0.49 |
| TIBC (mg/dl) | 244 ± 60 | 246 ± 65 | 239 ± 47 | 0.62 |
| Ferritin (ng/ml) | 233 ± 226 | 227 ± 206 | 248 ± 274 | 0.71 |
| CRP (mg/dl) | 0.6 ± 1.3 | 0.5 ± 1.2 | 0.9 ± 1.7 | 0.22 |
| Use of ACE inhibitors or ARBs (%) | 81 | 81 | 79 | 0.82 |
| Use of ESA (%) | 30 | 19 | 64 | <0.01 |
ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; CRP, C-reactive protein; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; ESA, erythropoiesis-stimulating agent; Fe, iron; HbA1c, glycosylated hemoglobin; HDL-C, high-density lipoprotein cholesterol; SBP, systolic blood pressure; T-Chol, total cholesterol; TIBC, total iron binding capacity.
n = 73.
Clinical Characteristics of Type 2 Diabetic Patients With and Without Anti-EPOR Antibodies
Patients’ serum samples were analyzed by ELISA. Anti-EPOR antibodies were detected in 26 of the 112 patients. Demographic and clinical findings were compared between the patients with and without anti-EPOR antibodies; selected findings are shown in Table 1. Patients with the antibodies were older than those without the antibodies (67.5 years vs. 61.5 years, respectively). Although the extent of proteinuria did not differ between both groups, renal function was significantly lower in the patients with the antibodies than in those without the antibodies, as indicated by the mean serum creatinine concentration and eGFR. In addition, the levels of total cholesterol and triglyceride were significantly decreased in the patients with anti-EPOR antibodies compared with those without the antibodies. Hb level was significantly lower in the patients with anti-EPOR antibodies than in those without the antibodies, although the number of reticulocytes was not significantly different. Erythropoiesis-stimulating agents were used more frequently in patients with the antibodies than in those without (64% vs. 19%, respectively; P < 0.01). No significant difference was found between the 2 groups for the other parameters examined, including duration of diabetes, HbA1c, systolic and diastolic blood pressures, high-density lipoprotein cholesterol, iron, total iron binding capacity, ferritin, and C-reactive protein at the time of work-up on admission and the use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers.
Stratified analysis of the patients separated into 2 groups according to baseline eGFR levels revealed that although mean baseline age and eGFR were similar in both groups, the difference in body mass index and Hb levels between patients with and without the antibodies was still observed in those with an eGFR <30 ml/min per 1.73 m2 (Supplementary Table S1). In addition, erythropoiesis-stimulating agents were used more frequently in patients with the antibodies than in those without in this subpopulation (94% vs. 45%, respectively).
Renal Pathological Characteristics of Type 2 Diabetic Patients With Anti-EPOR Antibodies
Renal biopsy findings were evaluated among the enrolled patients. Among the 51 patients who had been diagnosed histologically with diabetic nephropathy, an interstitial inflammation score of 2 was observed more frequently in the patients with anti-EPOR antibodies than in those without the antibodies, whereas glomerular and vascular lesions were not significantly different between both groups (Table 2).
Table 2.
Pathological features of patients with and without anti-erythropoietin receptor (EPOR) antibodies
| Pathological parameters | Anti-EPOR negative (n = 41) | Anti-EPOR positive (n = 10) | P value |
|---|---|---|---|
| Diffuse lesions scale 3 or 4 | 24 (58.5) | 3 (30.0) | 0.16 |
| Nodular lesion | 17 (41.5) | 3 (30.0) | 0.72 |
| Exudative lesion | 17 (41.5) | 3 (30.0) | 0.72 |
| Mesangiolysis | 16 (39.0) | 3 (30.0) | 0.73 |
| IFTA score 3 | 13 (31.7) | 3 (30.0) | 1 |
| Interstitial inflammation | 0.03 | ||
| Score 0 | 5 (12.2) | 0 (0.0) | |
| Score 1 | 28 (68.3) | 4 (40.0) | |
| Score 2 | 8 (19.5) | 6 (60.0) | |
| Arteriolar hyalinosis grade 2 or 3 | 8 (19.5) | 5 (50.0) | 0.10 |
| Arteriosclerosis score 2a | 6 (17.6) | 1 (16.7) | 1 |
IFTA, interstitial fibrosis and tubular atrophy.
n = 34 in the anti-EPOR negative group, n = 6 in the anti-EPOR positive group.
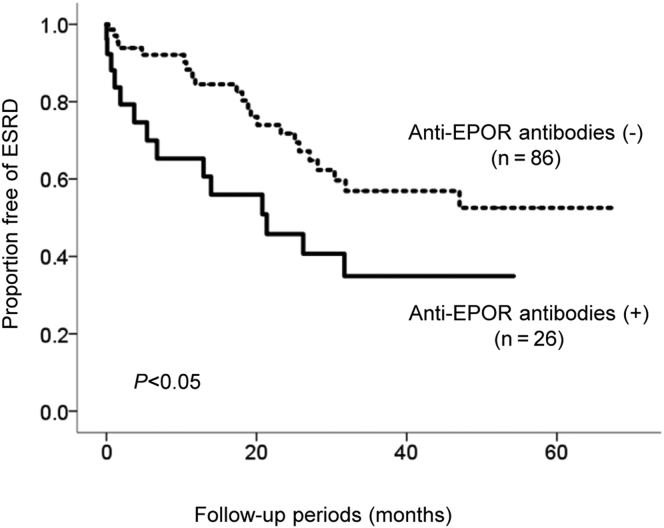
Outcome of Renal Function
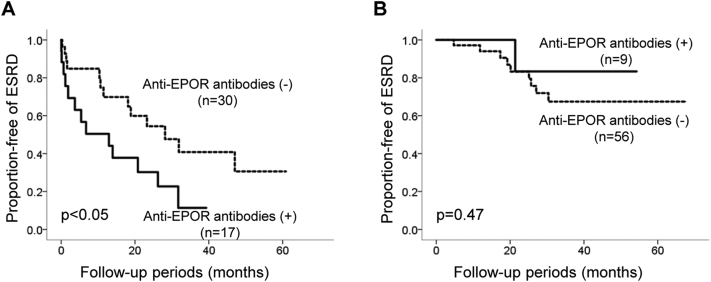
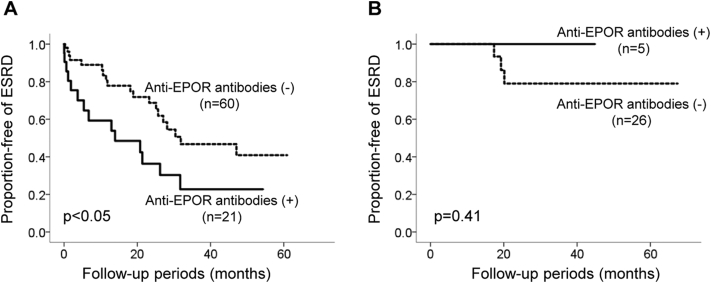
Follow-up data were available for renal events in the enrolled 112 patients. The mean follow-up during the study was 45.3 ± 47.4 months. The event-free rate of renal events in the patients with anti-EPOR antibodies was significantly lower than in those without the antibodies (Figure 1). When stratified into 2 groups according to baseline eGFR, this difference was observed in patients with a baseline eGFR <30 ml/min per 1.73 m2, whereas it was not detected in those with an eGFR >30 ml/min per 1.73 m2 (Supplementary Figure S1a and b). A difference in renal survival was also observed in patients with a baseline eGFR <60 ml/min per 1.73 m2, whereas there was a statistical difference in baseline eGFR (Supplementary Figure S2a and b, Supplementary Table S2).
Figure 1.
Renal outcome of the retrospective analysis of 112 type 2 diabetic patients with chronic kidney disease. Event-free rate of end-stage renal disease (ESRD) stratified by the presence or absence of autoantibodies to the erythropoietin receptor (EPOR) according to the Kaplan-Meier method. Dotted line, anti-EPOR−negative (n = 86); solid line, anti-EPOR−positive (n = 26). The mean follow-up was 45.3 ± 47.4 months. Differences between the groups were compared using a log-rank test.
Clinical Parameters Associated With Renal Events
The results of multivariate Cox proportional hazards regression analysis are shown in Table 3. The presence of anti-EPOR antibodies and high systolic blood pressure, proteinuria, and eGFR were independent risk factors for ESRD.
Table 3.
Parameters identified by multivariate Cox proportional hazards regression analysis associated with end-stage renal disease
| HR | 95% CI | P value | |
|---|---|---|---|
| Age per 1 yr | 1.04 | 1.01−1.07 | <0.05 |
| SBP per 1 mm Hg | 1.04 | 1.02−1.06 | <0.01 |
| Proteinuria per 1 g/d | 1.18 | 1.01−1.38 | <0.05 |
| eGFR per 1 ml/min per 1.73 m2 | 1.05 | 1.02−1.07 | <0.01 |
| Presence of anti-EPOR antibodies | 2.78 | 1.20−6.43 | <0.05 |
CI, confidence interval; eGFR, estimated glomerular filtration rate; EPOR, erythropoietin receptor; HR, hazard ratio; SBP, systolic blood pressure.
HRs are adjusted for sex, age, body mass index, SBP, proteinuria, eGFR, glycosylated hemoglobin; hemoglobin, and presence of anti-EPOR antibodies.
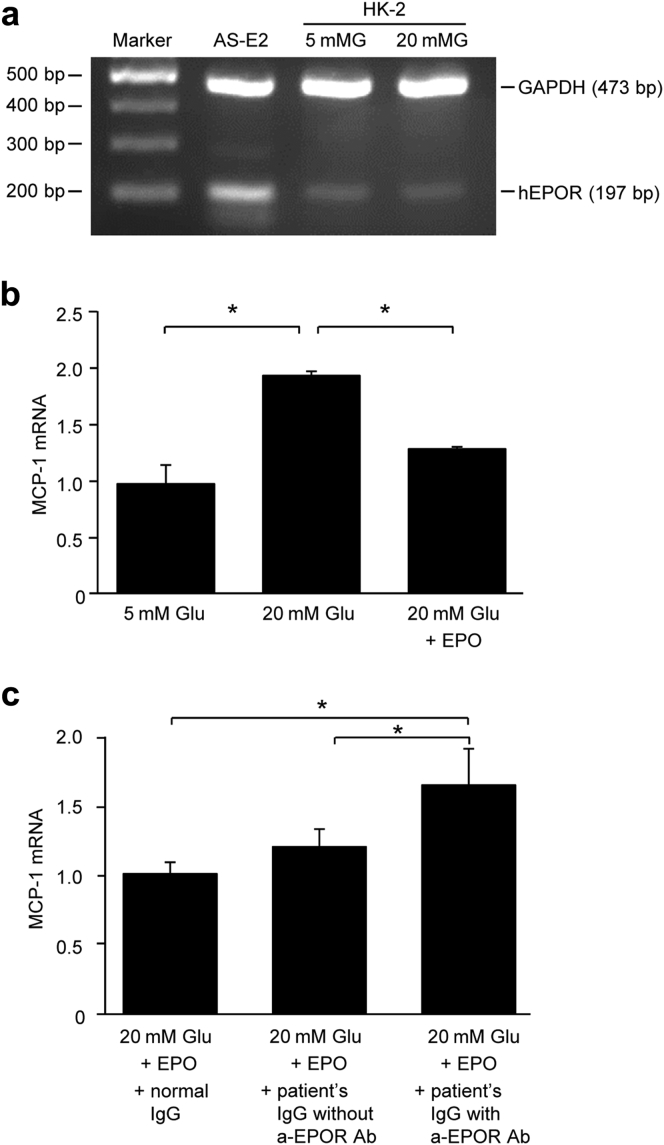
Effects of IgG Fractions Containing Anti-EPOR Antibodies on the Expression of MCP-1 mRNA in HK-2 Cells Under Diabetic Conditions
The effect of purified IgG fractions containing anti-EPOR antibodies on the expression of MCP-1 mRNA in HK-2 cells under high-glucose conditions was examined in vitro. The expression of EPOR was confirmed by RT-PCR (Figure 2a). Administration of 10 ng/ml EPO suppressed the high-glucose−induced upregulation of MCP-1 mRNA (Figure 2b). Furthermore, compared with IgG fractions purified from a healthy control subject and from a diabetic patient without anti-EPOR antibodies, IgG from a diabetic patient who was positive for the antibodies in the presence of EPO upregulated MCP-1 mRNA expression under the high glucose concentration (Figure 2c).
Figure 2.
Effect of erythropoietin (EPO) and IgG fractions containing anti-erythropoietin receptor (EPOR) antibodies on the expression of monocyte chemoattractant protein-1 (MCP-1) mRNA in HK-2 cells. (a) EPOR mRNA was assessed by reverse transcriptase polymerase chain reaction. (b) Stimulation of HK-2 cells with 20-mM D-glucose (20 mMG) increased the expression of MCP-1 mRNA, and EPO decreased it (n = 3). (c) Pretreatment with the IgG fraction from a patient with anti-EPOR antibodies <20 mM glucose upregulated the expression of MCP-1 mRNA (n = 3). All values are mean ± SD. *P < 0.05. a-EPOR Ab, anti-erythropoietin receptor antibodies; 5 mMG, 5-mM D-glucose; bp, base pair; GADPH, glyceraldehyde-3-phosphate dehydrogenase; hEPOR: human erythropoietin receptor.
Discussion
The present retrospective study demonstrated that serum anti-EPOR antibodies were detected in a subset of type 2 diabetic patients with CKD. Pathologically, the presence of antibodies was associated with increased interstitial inflammation. In addition, renal function declined more rapidly in patients with the antibodies than in those without the antibodies, and the presence of anti-EPOR antibodies was an additional risk factor for the progression of renal dysfunction.
The present study revealed that anti-EPOR antibodies were present in 23% of type 2 diabetic patients with CKD who did not have antiglutamic acid decarboxylase antibodies. The presence of autoantibodies to self-proteins in type 2 diabetes was suggested and reported previously. For example, 11 autoantibodies were identified using a high-density protein microarray among type 2 diabetic subjects in which the estimated prevalence was highest for autoantibodies to transaldolase-1 and mitochondrial ribosomal protein S7 (79% and 40%, respectively).21 In addition, autoantibodies to DNA–advanced glycation end product22 and apolipoprotein A-123 and B-10024 have also been detected. Among these, anti−apolipoprotein A-1 autoantibodies were present in up to 36% of patients with type 2 diabetes and cardiovascular disease.23 Although the detailed pathogenic mechanisms underlying the development of autoantibodies remain to be investigated, anti-EPOR antibodies might be additional autoantibodies that could be present in patients with type 2 diabetes and CKD.
Anti-EPOR antibodies were pathologically associated with renal interstitial inflammation in type 2 diabetic patients with CKD. The interstitial cell infiltration observed in diabetic kidneys is an important pathological finding because of its impact on renal prognosis7, 25 because it is also found in other etiologies of CKD.26 Inflammatory interstitial infiltrates include T lymphocytes and macrophages.25, 26 These inflammatory cells, tubular epithelial cells, and vascular endothelial cells reportedly express EPOR, and the proliferation and activation of T lymphocytes were modulated by EPO administration in previous studies.27, 28, 29, 30 In the present study, EPOR mRNA was detected in human proximal tubular cells, and EPO attenuated the expression of MCP-1 mRNA under a high glucose condition in vitro. In addition, IgG fractions containing anti-EPOR antibodies in the presence of EPO upregulated the expression of MCP-1 mRNA. Whether binding of anti-EPOR antibodies to EPOR between these cells occurs in the kidney, and whether the consequent nephrotoxicities are involved in the progression of renal damage via increased interstitial inflammation, even at areas without IFTA, remain to be investigated. However, anti-EPOR antibodies could be a useful biomarker for interstitial inflammation, which is associated with renal prognosis, in patients with type 2 diabetes.
The present study revealed that renal function in patients with anti-EPOR antibodies declined earlier than in patients without the antibodies. Furthermore, in multivariate analysis, the presence of anti-EPOR antibodies was selected as a significant risk factor for the decline of renal function. In this study, clinical parameters, including higher proteinuria and systolic blood pressure together with lower renal function, were confirmed as independent risk factors for ESRD in type 2 diabetic patients.7, 9, 10 In addition to the effects of anemia on the kidney, the present study and previous basic studies demonstrated that EPOR is expressed by resident renal cells, including endothelial cells and tubular epithelial cells.29, 30 Previous studies demonstrated that EPO has renoprotective effects through the suppression of inflammation, oxidative stress, and apoptosis of involved cells.31 Based on these non-erythropoietic activities of EPO, the presence of antibodies to EPOR was selected and considered as an independent explanatory factor from the levels of Hb in the present analysis. In the clinical setting, although clinical efficacy has yet to be validated, the administration of erythropoiesis-stimulating agents to patients with CKD has the potential to preserve renal function, as suggested by previous clinical and epidemiological studies.18, 32, 33 The findings of these studies suggest that renal function may be exacerbated by the blockade of the EPO–EPOR interaction via antibodies. Taken together, although the effect is relatively limited, anti-EPOR antibodies can serve as a serologic marker for the progression of renal injury in type 2 diabetic patients with CKD in combination with other known parameters, including albuminuria and proteinuria.
We noted several limitations in the present study. First, this study was dependent on collectable medical records because of its retrospective design. Second, limiting study enrollment to only patients who were admitted to our tertiary medical care institution for workup and treatment of an increased amount of proteinuria and/or kidney dysfunction during the course of diabetes likely created a bias. Third, treatment contents were not evaluated fully. These limitations might have placed significant constraints on the interpretation of the results, particularly those related to differences in renal outcome. However, clinical examinations concomitant with renal biopsy samples in these patients are important for understanding the pathophysiology of type 2 diabetes and its clinical outcome.
In conclusion, autoantibodies to EPOR may be involved in disease progression and may be a useful serologic marker for renal prognosis in type 2 diabetic patients with CKD. These potential biomarkers could help physicians diagnose high-risk patients and provide them more intensive care to prevent ESRD.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number JP16K09608 and a Grant-in-Aid for Practical Research Project for Renal Diseases from the Japan Agency for Medical Research and Development (Grant Number:15ek0310003h0001).
Contributions of the authors are as follows: AH performed the experiments and was involved in the interpretation of the results and preparation of the manuscript. KF assisted in the analysis. AK contributed to collecting clinical data. HY and TT performed and assisted with measurement of the antibodies by enzyme-linked immunosorbent assay. TT also performed the polymerase chain reaction. YI, NS, SK, and HN assisted in the analysis and were involved in the interpretation of the results. MS contributed to the collection of pathological data and to the interpretation of the results. TW initiated, organized, and designed the study, contributed to the analysis and interpretation of the data, and wrote the manuscript.
Footnotes
Figure S1. Renal outcome of type 2 diabetic patients according to baseline estimated glomerular filtration rates (eGFR) levels below and above 30 ml/min per 1.73 m2. Event-free rate of ESRD stratified by the presence or absence of autoantibodies to EPOR according to the Kaplan-Meier method. (a) Patients with baseline eGFR < 30 ml/min per1.73 m2. Dotted line, anti-EPOR-negative (n = 30); solid line, anti-EPOR-positive (n = 17). (b) Patients with baseline eGFR > 30 ml/min per1.73 m2. Dotted line, anti-EPOR-negative (n = 56); solid line, anti-EPOR-positive (n = 9). Differences between the groups were compared using a log-rank test.
Figure S2. Renal outcome of type 2 diabetic patients according to baseline estimated glomerular filtration rate (eGFR) levels below and above 60 ml/min per 1.73 m2. Event-free rate of ESRD stratified by the presence or absence of autoantibodies to EPOR according to the Kaplan-Meier method. (a) Patients with baseline eGFR < 60 ml/min per 1.73 m2. Dotted line, anti-EPOR-negative (n = 60); solid line, anti-EPOR-positive (n = 21). (b) Patients with baseline eGFR > 60 ml/min per 1.73 m2. Dotted line, anti-EPOR-negative (n = 26); solid line, anti-EPOR-positive (n = 5). Differences between the groups were compared using a log-rank test.
Table S1. Baseline characteristics of patients according to baseline estimated glomerular filtration rate (eGFR) levels below and above 30 ml/min per 1.73 m2.
Table S2. Baseline characteristics of patients according to baseline estimated glomerular filtration rate (eGFR) levels below and above 60 ml/min per 1.73 m2.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Figure S1.
Renal outcome of type 2 diabetic patients according to baseline estimated glomerular filtration rates (eGFR) levels below and above 30 ml/min per 1.73 m2. Event-free rate of ESRD stratified by the presence or absence of autoantibodies to EPOR according to the Kaplan-Meier method. (a) Patients with baseline eGFR < 30 ml/min per1.73 m2. Dotted line, anti-EPOR-negative (n = 30); solid line, anti-EPOR-positive (n = 17). (b) Patients with baseline eGFR > 30 ml/min per1.73 m2. Dotted line, anti-EPOR-negative (n = 56); solid line, anti-EPOR-positive (n = 9). Differences between the groups were compared using a log-rank test.
Figure S2.
Renal outcome of type 2 diabetic patients according to baseline estimated glomerular filtration rate (eGFR) levels below and above 60 ml/min per 1.73 m2. Event-free rate of ESRD stratified by the presence or absence of autoantibodies to EPOR according to the Kaplan-Meier method. (a) Patients with baseline eGFR < 60 ml/min per 1.73 m2. Dotted line, anti-EPOR-negative (n = 60); solid line, anti-EPOR-positive (n = 21). (b) Patients with baseline eGFR > 60 ml/min per 1.73 m2. Dotted line, anti-EPOR-negative (n = 26); solid line, anti-EPOR-positive (n = 5). Differences between the groups were compared using a log-rank test.
Baseline characteristics of patients according to baseline estimated glomerular filtration rate (eGFR) levels below and above 30 ml/min per 1.73 m2.
Baseline characteristics of patients according to baseline estimated glomerular filtration rate (eGFR) levels below and above 60 ml/min per 1.73 m2.
References
- 1.International Diabetes Federation. IDF diabetes around the world. Diabetes Atlas. 7th ed. Brussels, Belgium: International Diabetes Federation; 2015:14−16.
- 2.American Diabetes Association Standards of medical care in diabetes-2015. Diabetes Care. 2015;38(Suppl 1):S58–S60. [PubMed] [Google Scholar]
- 3.Tuttle K.R., Bakris G.L., Bilous R.W. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis. 2014;64:510–533. doi: 10.1053/j.ajkd.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 4.De Boer I.H., Rue T.C., Hall Y.N. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532–2539. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adler A.I., Stevens R.J., Manley S.E., UKPDS GROUP Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003;63:225–232. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- 6.Wada T., Haneda M., Furuichi K., Research Group of Diabetic Nephropathy, Ministry of Health, Labour, and Welfare of Japan Clinical impact of albuminuria and glomerular filtration rate on renal and cardiovascular events, and all-cause mortality in Japanese patients with type 2 diabetes. Clin Exp Nephrol. 2014;18:613–620. doi: 10.1007/s10157-013-0879-4. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu M., Furuichi K., Toyama T. Kanazawa Study Group for Renal Diseases and Hypertension. Long-term outcomes of Japanese type 2 diabetic patients with biopsy-proven diabetic nephropathy. Diabetes Care. 2013;36:3655–3662. doi: 10.2337/dc13-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toyama T., Furuichi K., Ninomiya T. The impacts of albuminuria and low eGFR on the risk of cardiovascular death, all-cause mortality, and renal events in diabetic patients: meta-analysis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0071810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keane W.F., Brenner B.M., de Zeeuw D., RENAAL Study Investigators The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int. 2003;63:1499–1507. doi: 10.1046/j.1523-1755.2003.00885.x. [DOI] [PubMed] [Google Scholar]
- 10.Rossing K., Christensen P.K., Hovind P. Progression of nephropathy in type 2 diabetic patients. Kidney Int. 2004;66:1596–1605. doi: 10.1111/j.1523-1755.2004.00925.x. [DOI] [PubMed] [Google Scholar]
- 11.Mehdi U., Toto R.D. Anemia, diabetes, and chronic kidney disease. Diabetes Care. 2009;32:1320–1326. doi: 10.2337/dc08-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hara A., Furuichi K., Higuchi M. Autoantibodies to erythropoietin receptor in patients with immune-mediated diseases: relationship to anaemia with erythroid hypoplasia. Br J Haematol. 2013;160:244–250. doi: 10.1111/bjh.12105. [DOI] [PubMed] [Google Scholar]
- 13.Hara A., Furuichi K., Yamahana J. Effect of autoantibodies to erythropoietin receptor in systemic lupus erythematosus with biopsy-proven lupus nephritis. J Rheumatol. 2016;43:1328–1334. doi: 10.3899/jrheum.151430. [DOI] [PubMed] [Google Scholar]
- 14.Matsuo S., Imai E., Horio M. Collaborators developing the Japanese equation for estimated GFR: revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 15.Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus. Seino Y., Nanjo K., Tajima N. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig. 2010;1:212–228. doi: 10.1111/j.2040-1124.2010.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tervaert T.W., Mooyaart A.L., Amann K., Renal Pathology Society Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21:556–563. doi: 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- 17.Anagnostou A., Liu Z., Steiner M. Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci U S A. 1994;91:3974–3978. doi: 10.1073/pnas.91.9.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuriyama S., Tomonari H., Yoshida H. Reversal of anemia by erythropoietin therapy retards the progression of chronic renal failure, especially in nondiabetic patients. Nephron. 1997;77:176–185. doi: 10.1159/000190270. [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki Y., Kuriyama K., Higuchi M. Establishment and characterization of a new erythropoietin-dependent acute myeloid leukemia cell line, AS-E2. Leukemia. 1997;11:1941–1949. doi: 10.1038/sj.leu.2400838. [DOI] [PubMed] [Google Scholar]
- 20.Schröppel B., Fischereder M., Wiese P. Expression of glucose transporters in human peritoneal mesothelial cells. Kidney Int. 1998;53:1278–1287. doi: 10.1046/j.1523-1755.1998.00899.x. [DOI] [PubMed] [Google Scholar]
- 21.Chang D.C., Piaggi P., Hanson R.L. Use of a high-density protein microarray to identify autoantibodies in subjects with type 2 diabetes mellitus and an HLA background associated with reduced insulin secretion. PLoS One. 2015;10:e0143551. doi: 10.1371/journal.pone.0143551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashraf J.M., Arfat M.Y., Arif Z. A clinical correlation of anti-DNA-AGE autoantibodies in type 2 diabetes mellitus with disease duration. Cell Immunol. 2015;293:74–79. doi: 10.1016/j.cellimm.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 23.El-Lebedy D., Rasheed E., Kafoury M. Anti-apolipoprotein A-1 autoantibodies as risk biomarker for cardiovascular diseases in type 2 diabetes mellitus. J Diabetes Complications. 2016;30:580–585. doi: 10.1016/j.jdiacomp.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Fredrikson G.N., Anand D.V., Hopkins D. Associations between autoantibodies against apolipoprotein B-100 peptides and vascular complications in patients with type 2 diabetes. Diabetologia. 2009;52:1426–1433. doi: 10.1007/s00125-009-1377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wada T., Furuichi K., Sakai N. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int. 2000;58:1492–1499. doi: 10.1046/j.1523-1755.2000.00311.x. [DOI] [PubMed] [Google Scholar]
- 26.Zeisberg M., Neilson E.G. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol. 2010;21:1819–1834. doi: 10.1681/ASN.2010080793. [DOI] [PubMed] [Google Scholar]
- 27.Cravedi P., Manrique J., Hanlon K.E. Immunosuppressive effects of erythropoietin on human alloreactive T cells. J Am Soc Nephrol. 2014;25:2003–2015. doi: 10.1681/ASN.2013090945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lisowska K.A., Debska-Slizień A., Bryl E. Erythropoietin receptor is expressed on human peripheral blood T and B lymphocytes and monocytes and is modulated by recombinant human erythropoietin treatment. Artif Organs. 2010;34:654–662. doi: 10.1111/j.1525-1594.2009.00948.x. [DOI] [PubMed] [Google Scholar]
- 29.De Beuf A., Hou X.H., D'Haese P.C. Epoetin delta reduces oxidative stress in primary human renal tubular cells. J Biomed Biotechnol. 2010;2010:395785. doi: 10.1155/2010/395785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beleslin-Cokic B.B., Cokic V.P., Yu X. Erythropoietin and hypoxia stimulate erythropoietin receptor and nitric oxide production by endothelial cells. Blood. 2004;104:2073–2080. doi: 10.1182/blood-2004-02-0744. [DOI] [PubMed] [Google Scholar]
- 31.Pearl R.G. Erythropoietin and organ protection: lessons from negative clinical trials. Crit Care. 2014;18:526. doi: 10.1186/s13054-014-0526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsubakihara Y., Gejyo F., Nishi S. High target hemoglobin with erythropoiesis-stimulating agents has advantages in the renal function of non-dialysis chronic kidney disease patients. Ther Apher Dial. 2012;16:529–540. doi: 10.1111/j.1744-9987.2012.01082.x. [DOI] [PubMed] [Google Scholar]
- 33.Cody J., Daly C., Campbell M. Recombinant human erythropoietin versus placebo or no treatment for the anaemia of chronic kidney disease in people not requiring dialysis. Cochrane Database Syst Rev. 2016;1:CD003266. doi: 10.1002/14651858.CD003266.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics of patients according to baseline estimated glomerular filtration rate (eGFR) levels below and above 30 ml/min per 1.73 m2.
Baseline characteristics of patients according to baseline estimated glomerular filtration rate (eGFR) levels below and above 60 ml/min per 1.73 m2.