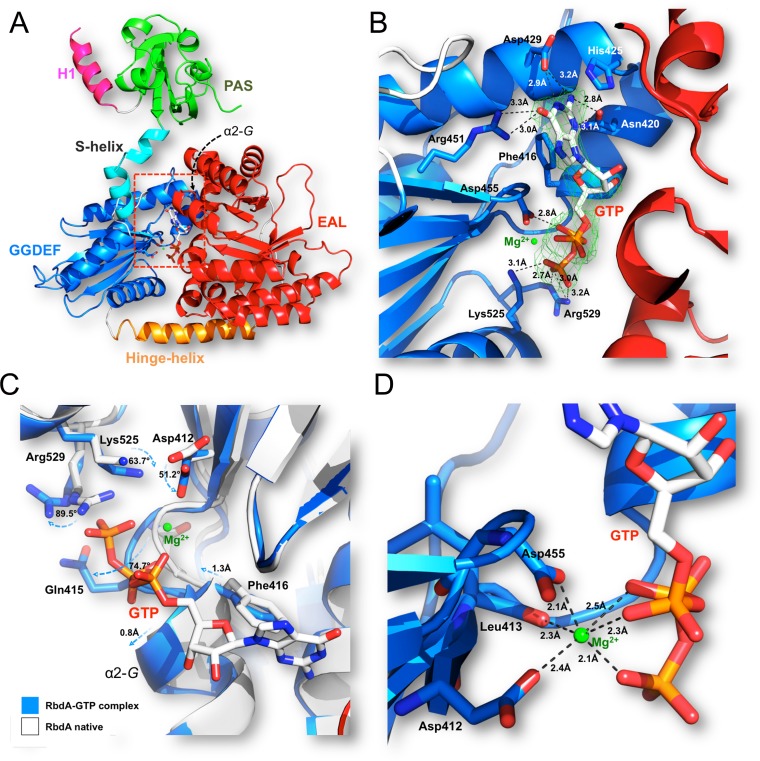
FIG 5.
Interaction between the GGDEF domain of cRbdA and the GTP allosteric activator of PDE activity. (A) Overall view of the cRbdA monomer, highlighting the location of the bound GTP molecule within the GGDEF domain. (B and D) Closeup views showing the atomic interactions between GTP (sticks) and residues of the A site of the GGDEF domain (blue) (B) and the Mg (green sphere) coordination shell (D). A difference electron density map with Fo-Fc Fourier coefficients, with the GTP moiety omitted from the calculation, is displayed at a level of 3σ. (C) Conformational changes in RbdA triggered by GTP binding. An overlay of the native (blue) and GTP-bound (white) cRbdA structures is shown. Residues near the phosphate tail of GTP, including in helix α2-G, and undergoing large displacements between the GTP-free and bound states are shown as sticks and labeled. A list of atomic interactions between the GGDEF domain and GTP is given in Table S2 in the supplemental material, and contacts between the EAL domain of cRbdA and c-di-GMP are shown in Table S3.