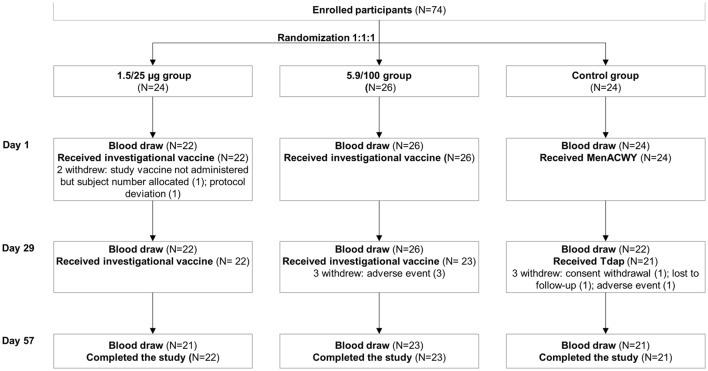
Figure 2.
Participant flowchart and timing of blood draws for immunogenicity analyses. N, number of participants; 1.5/25 μg, 5.9/100 μg monovalent Shigella sonnei 1790GAHB vaccine, with an O antigen/protein content of 1.5/25 and 5.9/100 μg, respectively; MenACWY, meningococcal vaccine against serogroups A, C, W, and Y; Tdap, tetanus, diphtheria, and acellular pertussis vaccine.