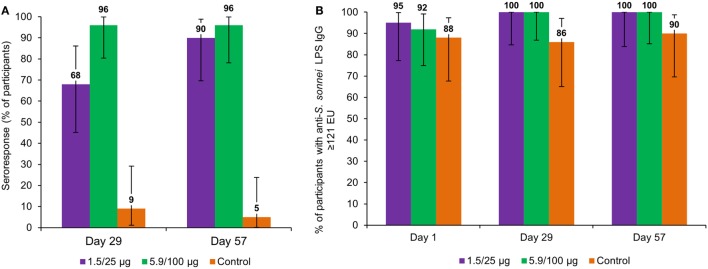
Figure 5.
Percentage of participants with seroresponse* (A) and anti-Shigella sonnei LPS IgG ≥121 EU (B), by timepoint (full analysis set for immunogenicity). LPS, lipopolysaccharide; IgG, immunoglobulin G; EU, enzyme-linked immunosorbent assay units; 1.5/25 μg, 5.9/100 μg, participants who received the 1790GAHB vaccine with an O antigen/protein content of 1.5/25 and 5.9/100 μg, respectively, at days 1 and 29; Control, participants who received meningococcal vaccine against serogroups A, C, W, and Y at day 1 and tetanus, diphtheria, and acellular pertussis vaccine at day 29. Note: *Seroresponse to vaccination was defined as an increase in the anti-S. sonnei LPS serum IgG level of ≥50% for participants with baseline levels >50 EU or an increase of ≥25 EU for participants with pre-vaccination levels ≤50 EU.