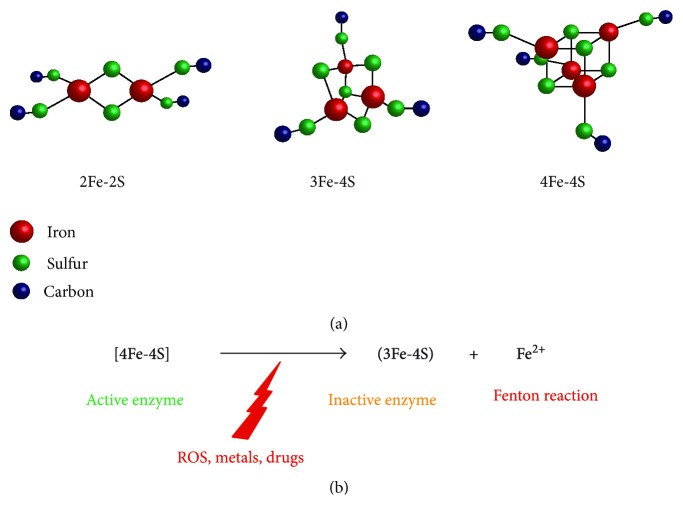
Figure 1.
Most common iron-sulfur structures. (a) Most common Fe-S clusters associated with proteins contain 2, 3, or 4 iron atoms. Oxidation states of the cluster are variable and can be [2Fe-2S]+ or [2Fe-2S]2+, [3Fe-4S]+, [3Fe-4S]0, [3Fe-4S]− or [3Fe-4S]2−, and [4Fe-4S]3+, [4Fe-4S]2+, [4Fe-4S]+, or [4Fe-4S]0. [3Fe-4S] clusters are most often considered as deriving from [4Fe-4S] clusters that have been oxidized by various cellular oxidants. Iron atoms are shown in red, sulfur atoms are shown in green, and carbon from cysteine residues are shown in dark blue. Coordination by histidine is not shown. (b) Conversion of [4Fe-4S] into [3Fe-4S] clusters is responsible for Fe2+ release and for enzyme inactivation. Fe2+ release might lead to Fenton reactions in the presence of hydrogen peroxide.