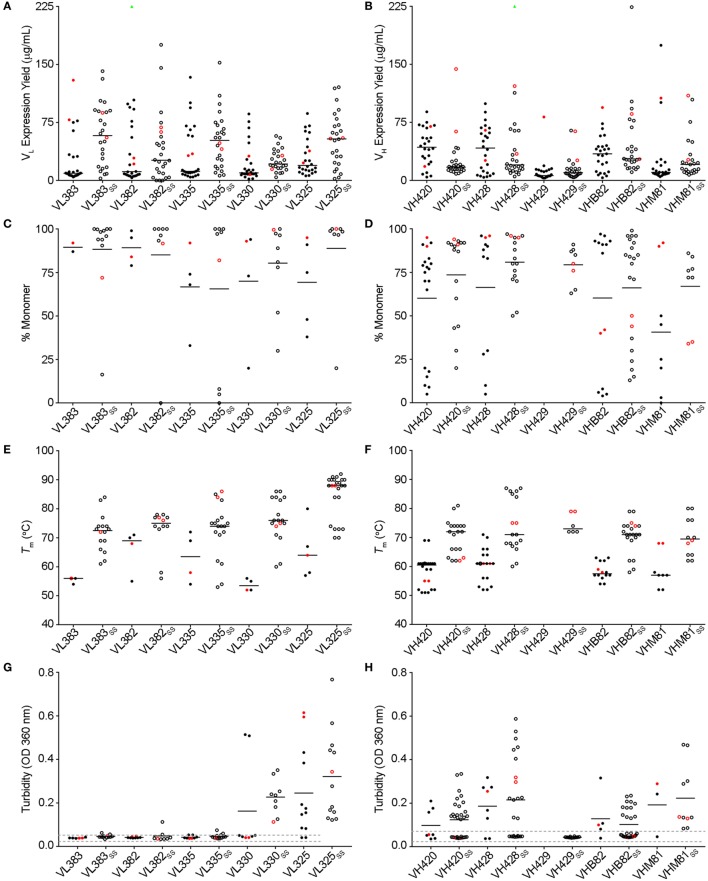
Figure 1.
Biophysical stability assessment of complementarity-determining region (CDR)-shuffled human VH/VL single-domain antibody (sdAb) variants. (A,B) Expression yields of CDR-shuffled VL (A) and VH (B) sdAbs from 5 mL overnight cultures, as determined by Bradford assay. Two outliers (VL382 + CDR set 9, expression yield 467.7 µg/mL and VH428SS + CDR set 2, 356.4 µg/mL) are indicated by green triangles. (C,D) Aggregation tendencies of CDR-shuffled VL (C) and VH (D) sdAbs, as determined by SEC-MALS. (E,F) Melting temperatures (Tms) of CDR-shuffled VL (E) and VH (F) sdAbs, as determined by thermal shift assay. (G,H) Turbidity upon thermal denaturation of CDR-shuffled VL (G) and VH (H) sdAbs, as measured by spectrophotometry at 360 nm wavelength. Dashed lines represent the range of turbidity measurements for unheated VH/VL sdAbs. The aggregation tendencies, Tms and turbidities of CDR-shuffled variants of VH429 were not characterized due to inadequate expression yields. Horizontal lines represent mean (C,D,G,H) or median (A,B,E,F) values and wild-type VH/VL sdAbs with unmodified CDRs are shown in red. Open and solid circles represent VH/VL sdAbs with, or without, a stabilizing exogenous disulfide linkage. The number of data points in each panel reflects whether the experiment was conducted in singlicate (C,E) or duplicate (A,B,D,F–H) and for panels (C–H), whether the CDR-shuffled variant expressed sufficiently for analysis.