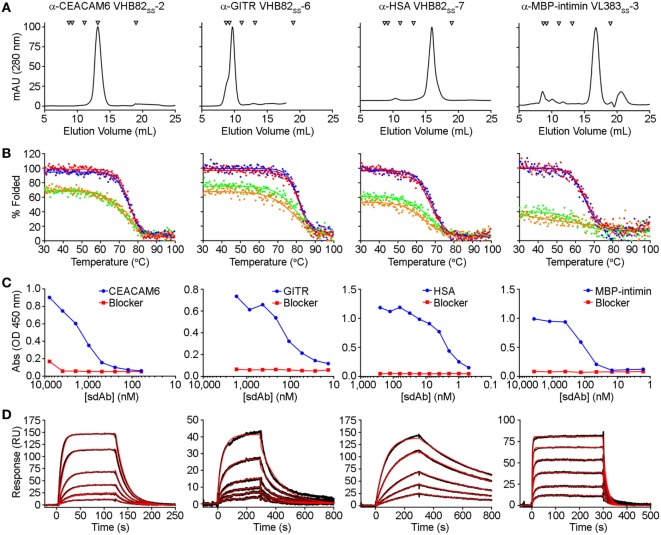
Figure 3.
Properties of selected antigen-specific single-domain antibodies (sdAbs) isolated from the VL383SS, VH428, and VHB82SS synthetic human VH/VL sdAb libraries. The single highest affinity sdAb against each target is shown, except for MBP-intimin, where VLSS-3 is shown instead of VLSS-4 or VLSS-5 because of better fit to a 1:1 binding model. (A) Size exclusion chromatography (SEC) profiles of representative antigen-specific human VH/VL sdAbs. Arrows show molecular mass standards, from left to right: thyroglobulin (670 kDa), gamma globulin (158 kDa), ovalbumin (44 kDa), myoglobin (17 kDa), and vitamin B12 (1.35 kDa). (B) Thermal unfolding of representative antigen-specific VH/VL sdAbs as determined by circular dichroism. Replicate unfolding curves are shown in red and blue; the sdAbs were then cooled to room temperature and remelted (shown in orange and green). (C) Titration ELISA of representative antigen-specific VH/VL sdAbs. Horseradish peroxidase mouse anti-c-Myc secondary antibody (clone 9E10, diluted 1:3,000) was used for detection. (D) Binding of antigen-specific VH/VL sdAbs to cognate antigen by SPR. Each antigen was immobilized on a CM5 sensor chip using amine coupling, then the indicated VH or VL sdAb was flowed over the surface at concentrations ranging from 25 to 1,000 nM (α-CEACAM6 VHB82SS-2), 25 to 500 nM (α-GITR VHB82SS-6), 2.5 to 50 nM (α-HSA VHB82SS-7), or 62.5 to 2,500 nM (α-MBP-intimin VL383SS-3). Black lines show data and red lines show fits.