Abstract
Pancreatic allograft thrombosis (PAT) remains the leading cause of nonimmunologic graft failure. Here, we propose a new computed tomography (CT) grading system of PAT to identify risk factors for allograft loss and outline a management algorithm by retrospective review of consecutive pancreatic transplantations between 2009 and 2014. Triple‐phase CT scans were graded independently by 2 radiologists as grade 0, no thrombosis; grade 1, peripheral thrombosis; grade 2, intermediate non‐occlusive thrombosis; and grade 3, central occlusive thrombosis. Twenty‐four (23.3%) of 103 recipients were diagnosed with PAT (including grade 1). Three (2.9%) grafts were lost due to portal vein thrombosis. On multivariate analysis, pancreas after simultaneous pancreas–kidney transplantation/solitary pancreatic transplantation, acute rejection, and CT findings of peripancreatic edema and/or inflammatory change were significant risk factors for PAT. Retrospective review of CT scans revealed more grade 1 and 2 thromboses than were initially reported. There was no significant difference in graft or patient survival, postoperative stay, or morbidity of recipients with grade 1 or 2 thrombosis who were or were not anticoagulated. Our data suggest that therapeutic anticoagulation is not necessary for grade 1 and 2 arterial and grade 1 venous thrombosis. The proposed grading system can assist clinicians in decision‐making and provide standardized reporting for future studies.
Keywords: clinical research/practice, coagulation and hemostasis, complication, graft survival, health services and outcomes research, pancreas/simultaneous pancreas‐kidney transplantation, thrombosis and thromboembolism
Short abstract
The Cambridge Pancreatic Allograft Thrombosis grading system may be used to standardize reports of thrombosis, identify patients who would benefit from therapeutic anticoagulation, provide prognostic information, and allow better comparison of reports from different centers for improved understanding and management of this common condition.
Abbreviations
- AIHA
autoimmune hemolytic anaemia
- BMI
body mass index
- CEUS
contrast‐enhanced ultrasound scan
- CI
confidence interval
- CIT
cold ischemia time
- CIV
common iliac vein
- CPR
cardiopulmonary resuscitation
- CT
computed tomography
- DBD
donation after brain death
- DCD
donation after circulatory death
- DVT
deep vein thrombosis
- EIA
external iliac artery
- GI
gastrointestinal
- INR
international normalized ratio
- IPTR
International Pancreas Transplant Registry
- IVC
inferior vena cava
- LOS
length of stay
- MRI
magnetic resonance imaging
- NT‐AC
no thrombosis–anticoagulated
- NT‐NAC
no thrombosis–not anticoagulated
- OR
odds ratio
- PAK
pancreas after kidney transplant
- PAM
pancreatica arteria magna
- PASPK
Pancreas after Simultaneous Pancreas and Kidney Transplantation
- PE
pulmonary embolism
- PGI2
prostacyclin
- PID
pelvic inflammatory disease
- PV
portal vein
- PVT
portal vein thrombosis
- RBC
red blood cell
- SA
splenic artery
- SBO
small bowel obstruction
- SMA
superior mesenteric artery
- SMV
superior mesenteric vein
- SPK
simultaneous pancreas–kidney transplant
- SPT
solitary pancreas transplantation
- SV
splenic vein
- T‐AC
thrombosis‐anticoagulated
- T‐NAC
thrombosis–not anticoagulated
- USS
ultrasound scan
- VAC
vacuum therapy
- VRE
vancomycin‐resistant Enterococcus
- WIT
warm ischemia time
1. INTRODUCTION
Pancreatic allograft thrombosis (PAT) is a potentially catastrophic complication and remains one of the leading causes of nonimmunologic allograft loss.1, 2 The incidence reported in the literature ranges from 1% to 40% and accounts for 29% of grafts lost within the first 6 months after transplantation.3, 4 Reported risk factors for PAT include donor age, body mass index (BMI), atherosclerosis, donation after circulatory death (DCD), death from cerebrovascular accident, and premortem severe hypotension.5, 6 Recipient risk factors include vascular disease, thrombophilic state, history of previous thrombotic events, and hypotension in the intraoperative or postoperative period.3 Other significant risk factors include type of preservation solution (reduced risk with UW), prolonged cold ischemia time (CIT), and technical factors during procurement and implantation.2, 7 Rate of graft loss secondary to PAT has been reported as 4% to 8% after simultaneous pancreas–kidney transplantation (SPK) and 10% to 12% after solitary pancreatic transplantation (SPT).8
Imaging plays a vital role in the evaluation of the transplanted pancreas. The modalities commonly used are Doppler ultrasound (USS), contrast‐enhanced ultrasound (CEUS), computed tomography (CT), CT angiography, magnetic resonance imaging (MRI), and MR angiography.9, 10, 11, 12 Vascular evaluation on imaging after pancreas transplantation is challenging due to the multiple anastomoses (4 or more), tortuous nature of graft vessels, and parenchymal edema related to surgery or pancreatitis. Overlying bowel gas may make ultrasound imaging suboptimal.13, 14, 15
Allograft thrombosis may affect arteries or veins, or sometimes both. Although there is no consensus on the classification or reporting of PAT, it is generally categorized as either complete or partial.16 Complete occlusion usually results in graft loss, unless thrombectomy or thrombolysis are undertaken soon after onset.17, 18, 19, 20 Conversely, there is some evidence to suggest a higher likelihood of pancreas allograft salvage with anticoagulation alone after partial thrombosis, although other interventions are occasionally required.21, 22 Outcomes after partial or complete occlusion of either splenic or superior mesenteric veins are better than complete occlusion of the main portal vein.23 Collateral arterial circulation may prevent infarction in cases of partial and occasionally complete PAT.24
There is currently no consensus on the optimal strategy for the prevention and management of PAT. Postoperative prophylaxis using low‐dose heparin infusion has been shown to reduce the thrombosis rate, but with a consequent increase in the number of bleeding events and need for repeat laparotomy.25, 26, 27
The aims of the current study were to analyze the incidence and management of PAT in a single center and to evaluate risk factors for PAT. We also propose a CT grading system of PAT and a management algorithm based on the grade of thrombosis.
2. MATERIALS AND METHODS
After institutional review board approval, a retrospective review was carried out of consecutive pancreas allograft transplants performed at our center between January 2009 and April 2014. Data regarding donor and recipient demographics, operative details, and outcomes were collected from a prospectively maintained electronic database. The outcomes reviewed were the incidence of PAT, graft and patient survival, postoperative complications, and length of postoperative hospital stay. Patients had workup for prothrombotic tendencies if a prior thrombotic event was identified.
The incidence, risk factors, and consequences of PAT were examined through the use of 2 methods. The first was a retrospective review of clinical data as recorded in the database during the original patient care episode, including incidence of thrombosis and graft and recipient outcomes (labeled “original diagnosis”). In the second stage, the first postoperative triple‐phase CT of the abdomen and pelvis (CTAP) of all pancreas transplant recipients were independently reviewed by 2 senior radiologists who were blinded to the initial CT report and the patient outcomes. Discrepancies were resolved by consensus. This “retrospective diagnosis” was used to definitively grade the thromboses and to formulate a management algorithm. The pancreatic allograft arteries and veins were graded as per the “new” grading system (Table 1). Representative cross‐sectional images demonstrating the different grades of thrombosis are shown in Figure 1.
Table 1.
Pancreatic allograft thrombosis grading system
| Grade 0 | No thrombosis |
| Grade 1 | Peripheral thrombosisa |
| Grade 2 | Intermediate nonocclusive thrombosisb , c |
| Grade 3 | Central occlusive thrombosis |
The allograft arteries and veins on triple‐phase CT scans were graded independently by 2 radiologists.
Grade 1 thrombosis: Thrombus lies in the very distal vessel at the transected margin of the superior mesenteric vein (SMV)/splenic vein (SV) or superior mesenteric artery (SMA)/SA and lies in a single branch only, without encroachment into the main trunk of the vessel.
Grade 2 venous: Thrombus extending into parenchymal vessels/main trunk of the SMV or SV to the SMV/SV confluence but not into the portal vein.
Grade 2 arterial: Thrombus extending into the main trunk of the SMA/SA to the “Y” graft but not into the Y graft.
Figure 1.
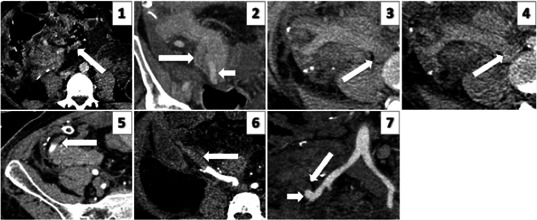
Representative cross‐sectional images demonstrating grades 1‐3 of venous and arterial pancreas allograft thrombosis. IMAGE 1: Grade 1 venous thrombus: axial image of a portal phase study demonstrating non‐occlusive thrombus (arrow) within an SMV tributary within fat, at the transected margin of the graft. IMAGE 2: Grade 2 venous thrombus: coronal reformatted image of a portal phase study demonstrating non‐occlusive thrombus extending along the splenic vein within the body of graft pancreas (arrow). Adjacent patent splenic artery (short arrow). IMAGE 3: Grade 3 venous thrombus: unenhanced axial image demonstrating acute hyper‐dense thrombus in an expanded SMV. IMAGE 4: Grade 3 venous thrombus: portal phase axial image demonstrating thrombus in the SMV extending into the IVC (arrow). This component is seen as hypo‐dense within otherwise opacified cava. IMAGE 5: Grade 1 arterial thrombus: axial arterial phase image demonstrating minimal thrombus in the distal splenic artery (arrow). IMAGE 6: Grade 2 arterial thrombus: axial arterial phase image demonstrating thrombus extending into the mid SMA. IMAGE 7: Grade 3 arterial thrombus: (image not from current series; obtained from a library) arterial phase coronal reformatted image demonstrating acute occlusive thrombus expanding the Y graft and SMA (arrow). Enhancement of the residual patent proximal Y graft stump (short arrow)
Acute rejection was defined as rejection on kidney or pancreas biopsy. Graft pancreatitis was defined as peripancreatic edema and/or inflammatory change on CT scanning.
2.1. Operative technique
Back‐bench preparation of the pancreatic graft involved removal of spleen, ligation of all the distal mesenteric vessels, and anastomosis of a donor iliac Y‐graft to the graft superior mesenteric and splenic arteries. The pancreas was placed in the right iliac fossa intraperitoneally through a midline incision, with anastomosis of the portal vein to the inferior vena cava and the donor iliac artery Y‐graft to the recipient common iliac artery. Enteric drainage was via a Roux‐en‐Y duodenoenterostomy. Feeding jejunostomy was inserted in most cases, and prophylactic appendicectomy and cholecystectomy (the latter, if preoperative USS demonstrated sludge/gallstones) were performed. All recipients were started on intravenous epoprostenol (Flolan®) 4.0 ng/kg/min immediately after reperfusion and continued for a period of 5 days or discontinued earlier in case of hemodynamic instability or bleeding. Those patients who were not taking aspirin 75 mg once daily were started on it at discharge or earlier if platelet count was greater than 500 × 109/L. The standard immunosuppression protocol was induction with alemtuzumab (CD52 monoclonal antibody; Campath 1H®) 30 mg subcutaneously. Maintenance immunosuppression was steroid free with tacrolimus 0.05 mg/kg twice daily started on the first postoperative day (trough concentration 8‐12 ng/mL) and mycophenolate mofetil started at 500 mg twice daily on postoperative day 7.
2.2. CT scanning
Triple‐phase CTAP was performed using 64‐ or 256‐section CT systems (Siemen AG Healthcare, Camberley, Surrey, UK). Unenhanced imaging of the abdomen was performed to localize the pancreatic graft, followed by early arterial phase imaging of the pancreatic and renal grafts and portal phase imaging of the abdomen and pelvis. We administered 100 mL of iopamidol nonionic contrast (Niopam 300; Bracco, High Wycombe, Bucks, UK) at a rate of 3 mL per second. Images were reconstructed and reviewed at 2‐mm section thickness on the picture archive and communication system (PACS GE). The triple‐phase CT scans were performed for clinical/biochemical reasons, and no routine or protocol scans were performed. Intravenous fluid rehydration was considered in recipients with a raised creatinine of greater than 150 μmol/L. Routine USS is performed to evaluate the renal allograft; routine USS of the pancreatic graft is not performed in our unit.
2.3. Statistical analysis
Categorical variables are expressed as frequency (%), and continuous variables as median (range). Categorical variables were analyzed by use of Fisher's exact or χ2 test, and continuous variables were analyzed by unpaired student t test, Mann‐Whitney U test, or other nonparametric tests. Bonferroni correction was used for multiple comparisons. The variables significant on univariate analysis were subjected to multivariate logistic regression analysis. Kaplan‐Meier survival curves were studied by using log‐rank statistics (SPSS 20.0; SPSS Institute, Chicago, IL, USA).
3. RESULTS
One hundred three consecutive pancreatic transplantations were performed during the study period. The mean follow‐up period was 53.7 months (range 21.7‐83.3 months). Twenty‐four (23.3%) recipients were diagnosed with PAT (original diagnosis), of whom 4 patients had this diagnosis confirmed on laparotomy, while 1 patient had a negative laparotomy. The thromboses were initially reported as arterial (n = 11, 10.7%), venous (n = 9, 8.7%) or both (n = 4, 3.9%). Of the arterial thromboses, 14 were grade 1 or 2 and 1 was grade 3. Of the venous thromboses, 8 were grade 1 or 2 and 5 were grade 3. Three patients required intraoperative revision of anastomosis for thrombosis during the index transplant operation, 2 of whom developed postoperative thrombosis and hence were included in the thrombosis group.
The patients who had thrombosis as their original diagnosis (n = 24, 23.3%) were compared with those who did not have thrombosis (n = 79, 76.7%) (Tables 2, 3, 4, 5).
Table 2.
Donor characteristics
| Thrombosis group (n = 24) | No‐thrombosis group (n = 79) | P valuea | |
|---|---|---|---|
| Age (y) | 41 (12‐59) | 37 (7‐64) | .548 |
| Gender (male) | 15 (62.5%) | 34 (43.0%) | .107 |
| White ethnicity | 21 (87.5%) | 71 (90.0%) | .715 |
| Body mass index (kg/m2) | 23 (15‐28) | 23 (17‐33) | .947 |
| Weight (kg) | 70 (33‐90) | 70 (44‐96) | .960 |
| Donor type (DBD) | 19 (79.2%) | 50 (63.3%) | .215 |
| Donor cause of death | |||
| Traumatic brain injury | 9 (37.5%) | 21 (26.6%) | .315 |
| Intracranial hemorrhage | 7 (29.2%) | 26 (32.9%) | .807 |
| Anoxic brain damage | 5 (20.8%) | 15 (18.9%) | 1.000 |
| Cerebral infarct | 2 (8.3%) | 2 (2.5%) | .231 |
| Central nervous system infection | 1 (4.2%) | 4 (5.1%) | 1.000 |
| Brain tumor | 0 (0.0%) | 5 (6.3%) | .588 |
| Other causes (pneumonia, sepsis) | 0 (0.0%) | 6 (7.7%) | .332 |
| Hemoglobin (g/L) | 117 (74‐160) | 117 (63‐161) | .629 |
| Platelets (×109/L) | 194 (69‐303) | 197 (7‐707) | .718 |
| Serum amylase (units/L) | 58 (8‐1075) | 45 (6‐338) | .721 |
| Smoking history (yes) | 13 (54.2%) | 42 (53.2%) | 1.000 |
| Inotrope use at referral (yes) | 20 (83.3%) | 60 (75.9%) | .580 |
| Previous thrombotic events | 1 (4.2%) [DVT] | 0 (0.0%) | .262 |
| Anti‐inflammatory drug use | 1 (4.2%) | 0 (0.0%) | .262 |
Donor demographics were similar in the thrombosis and no‐thrombosis groups. Continuous variables are expressed as median (range) and categorical variables as frequencies (percentages).
DVT, deep vein thrombosis.
P value considered to be significant if <.025 (Bonferroni corrected 0.05/2 = 0.025).
Table 3.
Recipient characteristics
| Thrombosis group (n = 24) | No‐thrombosis group (n = 79) | P value* | |
|---|---|---|---|
| Age (y) | 43 (32‐51) | 44 (24‐58) | .468 |
| Gender (male) | 17 (70.8%) | 47 (59.5%) | .348 |
| Body mass index (kg/m2) | 25 (19‐30) | 26 (18‐30) | .691 |
| Weight (kg) | 73 (56‐94) | 70 (45‐102) | .402 |
| Dialysis type | |||
| Predialysis | 9 (37.5%) | 35 (44.3%) | .641 |
| Peritoneal dialysis | 2 (8.3%) | 15 (19.0%) | .347 |
| Hemodialysis | 12 (50.0%) | 31 (36.7%) | .357 |
| Hemodialysis/peritoneal dialysis | 1 (4.2%) | 0 (0.0%) | .233 |
| Duration of dialysis (mo) | 10.5 (0‐154) | 5 (0‐114) | .343 |
| Total HLA‐A, ‐B, ‐DR mismatches | |||
| 0‐1 | 0 (0.0%) | 2 (2.6%) | 1.000 |
| 2‐4 | 16 (66.7%) | 49 (62.0%) | .810 |
| 5‐6 | 8 (33.3%) | 28 (35.4%) | 1.000 |
| Previous myocardial infarction | 5 (20.8%) | 16 (20.2%) | 1.000 |
| Previous thrombotic events |
3 (12.5%) [DVTs 2; pancreas graft venous thrombosis 1] |
5 (6.3%) [DVT 3; PE 2] |
.385 |
| History of hypercoagulability | 2 (8.3%) | 8 (10.1%) | 1.000 |
| Anti‐inflammatory drug use | 1 (4.2%) | 5 (6.3%) | 1.000 |
| Preoperative hemoglobin | 121 (84‐154) | 113 (79‐150) | .065 |
| Preoperative platelets | 242 (135‐429) | 236 (110‐605) | .823 |
| Preoperative urea | 13.5 (5.9‐31.0) | 19.0 (5.3‐42.0) | .311 |
| Preoperative creatinine | 485 (98‐981) | 499 (175‐1142) | .823 |
| Previous failed transplants |
3 (12.5%) [all SPKs] |
4 (5.1%) [2 SPK and 2 kidney alone transplants] |
.349 |
Continuous variables are expressed as median (ranges) and categorical variables as frequencies (percentages).
DVT, deep vein thrombosis; PE, pulmonary embolism; SPK, simultaneous pancreas–kidney transplant.
Table 4.
Operative characteristics
| Thrombosis group (n = 24) | No‐thrombosis group (n = 79) | P valueb | |
|---|---|---|---|
| Year of transplantation | |||
| 2009 | 2 (8.3%) | 17 (21.5%) | |
| 2010 | 8 (33.3%) | 22 (27.8%) | 1.000 |
| 2011 | 1 (4.2%) | 13 (16.4%) | .100 |
| 2012 | 3 (12.5%) | 9 (11.4%) | 1.000 |
| 2013 | 8 (33.3%) | 15 (19.0%) | .280 |
| 2014 | 2 (8.3%) | 3 (3.8%) | .602 |
| Organ transplanted | |||
| SPK | 18 (75.0%) | 78 (98.7%) | <.001 |
| PASPK/PAK | 4 (25.0%) [3 PASPK and 1 PAK] | 1 (1.3%) [PASPK] | – |
| Pancreas artery reconstruction | |||
| Donor Y‐graft (SMA and SA) | 20 (83.3%) | 75 (95.0%) | .083 |
| Donor Y‐graft plus PAM anastomosis |
2 (8.3%) [onto Y‐graft; using donor splenic artery segment] |
2 (2.5%) | .231 |
| Donor Y‐graft (aberrant artery from coeliac. SMA and coeliac onto Y‐graft) | 1 (4.2%) | 0 (0.0%) | .233 |
| No donor Y‐graft |
1 (4.2%) [Splenic onto SMA] |
2 (2.5%) [donor coeliac artery from SMA, SMA and SA onto EIA and end‐side anastomosis] |
.553 |
| Pancreas PV reconstruction (yes) | 1 (4.2%) | 0 (0.0%) | .233 |
| Arterial conduit implant site | |||
| Aorta | 0 (0.0%) | 1 (1.3%) | 1.000 |
| Common iliac artery | 22 (91.7%) | 56 (70.8%) | .055 |
| External iliac artery | 2 (8.3%) | 21 (26.6%) | .091 |
| Internal iliac artery | 0 (0.0%) | 1 (1.3%) | 1.000 |
| Portal vein implant site | |||
| IVC | 23 (95.8%) | 78 (98.7%) | .413 |
| Common iliac vein | 1 (4.2%) [right CIV] | 0 (0.0%) | .233 |
| External iliac vein | 0 (0.0%) | 1 (0.0%) | 1.000 |
| Intraoperative thrombosis requiring revision of anastomosis |
2 (8.3%) [portal vein anastomosis] |
1 (1.3%) [arterial anastomosis] |
.135 |
| Donor WIT for DCD organs (min) | 12 (7‐13) | 13 (3‐134) | .104 |
| Recipient WIT (min) | 40 (25‐79) | 37 (19‐57) | .103 |
| Cold ischemia time (CIT) (min) | 608 (423‐817) | 583 (271‐895) | .393 |
| CIT‐DBD (min)a | 624 (423‐805) [19] | 599 (271‐895) [50] | .400 |
| CIT‐DCD (min)a | 577 (455‐817) [5] | 537 (297‐773) [29) | .524 |
| Intraoperative blood transfusion | 7 (29.2%) | 23 (29.1%) | .588 |
Continuous variables are expressed as median (range) and categorical variables as frequencies (percentages).
Pancreas after SPK or pancreas after kidney (PASPK/PAK) was the only significant risk factor for allograft thrombosis.
CIT, cold ischemia time; CIV, common iliac vein; DCD, donation after circulatory death; EIA, external iliac artery; IVC, inferior vena cava; PAK, pancreas after kidney transplant; PAM, pancreatica arteria magna; PV, portal vein; SA, splenic artery; SMA, superior mesenteric artery; SPK, simultaneous pancreas–kidney transplant; WIT, warm ischemia time.
Number of patients given in brackets.
P value considered to be significant if <.025 (Bonferroni corrected 0.05/2 = 0.025). P values that are significant are highlighted in bold.
Table 5.
Recipient outcomes
| Thrombosis group (n = 24) | No‐thrombosis group (n = 79) | P value* | |
|---|---|---|---|
| Number of RBC units transfused (intraoperative plus postoperative) | 4 (2‐7) | 3 (1‐9) | .513 |
| Peak serum amylase (units/L) [within 3 days postoperative] | 128 (8‐832) | 134 (3‐512) | .823 |
| Time of thrombosis diagnosis after transplantation (days) | 5 (0‐120) | Not applicable | – |
| Therapeutic anticoagulation posttransplantation | 24 (100%) | Not applicable | – |
| Surgical reexploration for thrombosis |
5 (20.8%) (1 arterial, 3 venous, and 1 arterial and venous thrombosis) |
Not applicable | – |
| Surgical reexploration for bleeding |
8 (33.3%) [3 preanticoagulation and 5 postanticoagulation; 1 patient had 2 explorations postanticoagulation] |
12 (15.2%) [1 patient had 2 explorations] |
.074 |
| Length of postoperative stay (days) | 21 (10‐57) | 15 (6‐305) | .001 |
| Acute rejection | 8 (33.3%) | 7 (8.9%) | .006 |
| Peripancreatic edema and/or inflammatory changes on CT | 12 (50.0%) | 11 (13.9%) | <.001 |
| Indication for first postoperative CT | |||
| Hyperglycemia | 10 (41.7%) | 22 (21.0%) | .216 |
| Suspected bleeding | 5 (20.8%) | 14 (17.7%) | .767 |
| Abdominal pain | 3 (12.5%) | 6 (4.8%) | .432 |
| Abdominal distention | 1 (4.1%) | 3 (4.8%) | 1.000 |
| Sepsis | 3 (12.5%) | 17 (22.6%) | .393 |
| Increasing amylase/lipase | 0 (0.0%) | 6 (6.5%) | .331 |
| Increasing drain amylase | 0 (0.0%) | 1 (1.6%) | 1.000 |
| Number of postoperative days for first CT (days) | 5 (1‐41) | 5 (0‐632) | .862 |
| Total number of CT scans during the study period | 4 (0‐15) | 2 (0‐18) | .003 |
| Postoperative complications (30‐day) | 13 Patients/21 complications | 31 Patients/39 complications | .241 |
| Wound infection | 3 | 3 | |
| Perinephric collection – USS drainage | 1 | 3 | |
| Abdominal collection – USS drainage | 1 | 2 | |
| Graft pancreatitis | 1 | 1 | |
| Pneumonia | 2 | 6 | |
| VRE bacteremia | 1 | 0 | |
| Relaparotomy for SBO | 1 | 0 | |
| Relaparotomy for SB ischemia | 0 | 1 | |
| Relaparotomy for internal hernia | 0 | 1 | |
| Relaparotomy for GI bleed | 0 | 1 | |
| Line‐related sepsis | 3 | 2 | |
| Wound dehiscence (VAC therapy) | 1 | 0 | |
| Wound dehiscence (skin graft/VAC) | 0 | 1 | |
| Complete wound dehiscence (repaired) | 0 | 1 | |
| Graft pancreatectomy | 3 | 0 | |
| Graft nephrectomy | 1 | 0 | |
| Clostridium difficle infection | 1 | 2 | |
| Left brachial plexus neuralgia | 1 | 0 | |
| Urinary tract infection | 0 | 7 | |
| Pulmonary embolism | 0 | 1 | |
| Cardiac arrest needing CPR | 0 | 1 | |
| Laparotomy for pneumatosis coli | 0 | 1 | |
| Spinal cord infarct – paraplegia | 0 | 1 | |
| Prostatic abscess drained | 0 | 1 | |
| Abdominal compartment syndrome | 0 | 1 | |
| Donor duodenum bleeding embolized | 0 | 1 | |
| Reactivation of PID (tubo‐ovarian abscess) | 0 | 1 | |
| Radial artery thrombosis secondary to indwelling catheter | 1 | 0 | |
| Renal allograft outcome – creatinine at the end of study follow‐up (μmol/L) | 111 (50‐353) | 99 (67‐342) | .605 |
| Graft loss |
6 (25.0%) [3 venous thrombosis, 1 pancreatitis with abscess formation and 2 chronic rejection] |
3 (3.8%) [1 chronic rejection, 1 graft vasculopathy, 1 immunological recurrence of type 1diabetes mellitus] |
<.0001 ** |
| Patient death |
2 (8.3%) [1 Unexplained sudden cardiac event, 1 unknown cause] |
4 (5.1%) [1 Ischemic small bowel, 1 gastric adenocarcinoma, 1 metastatic esophageal cancer, and 1 AIHA] |
.436** |
Continuous variables are expressed as median (range) and categorical variables as frequencies (percentages).
AIHA, autoimmune hemolytic anaemia; CPR, cardiopulmonary resuscitation; CT, computed tomography; GI, gastrointestinal; PE, pulmonary embolism; PID, pelvic inflammatory disease; PV, portal vein; RBC, red blood cell; SBO, small bowel obstruction; USS, ultrasound scan; VAC, vacuum therapy; VRE, vancomycin‐resistant Enterococcus.
*P value considered to be significant if <.025 (Bonferroni corrected 0.05/2 = 0.025). P values that are significant are highlighted in bold.
**Log‐rank (Mantel‐Cox) P value from survival analysis.
3.1. Donor characteristics
There was no difference between the 2 groups in terms of donor age, sex, ethnicity, weight, and BMI (Table 2). There were numerically more donation after brainstem death (DBD) donors in the thrombosis group (n = 19, 79.2%) compared with the no‐thrombosis group (n = 50, 63.3%), but this was not statistically significant (P = .107).
3.2. Recipient characteristics
There was no difference between the 2 groups in terms of recipient age, sex, weight, BMI, or previous thrombotic events (Table 3). Three (12.5%) patients in the thrombosis group had previous failed transplants (all SPK pancreases: 2 failed due to the recurrence of diabetes and 1 due to venous thrombosis) in comparison to 4 patients (5.1%) in the no‐thrombosis group (2 SPKs [lost due to venous thrombosis and chronic rejection] and 2 kidney‐alone transplantations) (P = .349).
3.3. Operative characteristics
In total, 4 (80%) of 5 pancreas after kidney or SPK (PAK/PASPK) transplants had thrombosis post‐operatively (P < .001) (Table 4). Only 1 recipient had portal vein reconstruction at the time of transplantation using an 8‐mm extension graft; he subsequently developed portal venous thrombosis. Two (8.3%) patients in the thrombosis group had intraoperative thrombosis, and the portal vein anastomoses were redone, in comparison to 1 patient (1.3%) in the no‐thrombosis group in whom the arterial anastomosis was redone (P = .135).
3.4. Recipient outcomes
Recipient outcomes for the 2 groups are shown in Table 5. The retrospective grading of CTs in patients with the original diagnosis of thrombosis were arterial thrombosis – grade 1 (n = 10), grade 2 (n = 4) and grade 3 (n = 1); and venous thrombosis – grade 1 (n = 5), grade 2 (n = 3) and grade 3 (n = 4). The median time of thrombosis diagnosis after transplantation was 5 days (range 0‐120 days). Of the 24 patients with an original diagnosis of thrombosis, 23 had a CT‐based diagnosis and 1 patient underwent surgical exploration without a CT scan. This patient, who was noted to be at high risk of venous thrombosis at the initial implant surgery and was anticoagulated in the immediate postoperative period, was reexplored on postoperative day 1 with removal of splenic vein thrombus. With continuing hyperglycemia and worsening lactate, the patient was reexplored again at 48 hours posttransplantation, and graft pancreatectomy was performed.
All patients with an original diagnosis of thrombosis were therapeutically anticoagulated with dalteparin (140 units/kg/day split into 2 doses at 12‐hour interval) in the first instance followed by warfarin for a period of 3 months (target INR 2‐2.5). Patients who underwent reexploration were anticoagulated after exploration surgery, unless graft pancreatectomy (3 patients) was performed.
There were no contrast‐induced allergic reactions in our study cohort. Eighty‐one patients had at least 1 postoperative CT during the study period. Serial creatinine results were available for 67 of these patients. Twelve patients had an acute rise in creatinine after CT scans, in keeping with contrast‐induced acute kidney injury (CI AKI), although 7 patients were receiving dialysis at the time. An alternative possible explanation for the rise in creatinine was identified in all 5 patients: 1 patient was diagnosed with rejection, 1 with hemorrhage, 1 with renal arterial flow abnormality on USS, and 2 with abdominal pain ± pyrexia. Renal function recovered in all 12 patients with rehydration.
3.4.1. Arterial thrombosis
One patient with grade 1 arterial thrombosis and extrinsic compression of the portal vein on CT was reexplored and no thrombus was seen within the artery, but there was severe pancreatitis of the allograft. The patient subsequently had a graft pancreatectomy performed on postoperative day 46 due to abscess formation and severe pancreatitis. One other patient with grade 1 arterial thrombosis (with grade 3 PVT) was reexplored, and the PV thrombus was removed. The graft failed on postoperative day 144 due to chronic rejection (biopsy proven). All other patients with arterial thrombosis (including grade 2 [n = 4] and grade 3 [n = 1]) were anticoagulated and had functioning grafts at the end of study follow‐up. The grade 3 arterial thrombosis patient's CT scan on retrospective review was reported as no thrombosis and only short‐segment stricture of splenic artery.
3.4.2. Venous thrombosis
All patients with grade 1 (n = 5) venous thrombosis were anticoagulated and had functioning grafts at the end of study follow‐up. The only patient who had a PV extension graft had a CT diagnosis of grade 2 venous thrombosis on postoperative day 5. Reexploration and thrombectomy did not improve graft perfusion, and pancreatectomy was performed. Another patient with grade 2 venous thrombosis was reexplored on postoperative day 1 and portal vein thrombus was removed; the graft was functioning at the end of study follow‐up. One other patient with grade 3 venous thrombosis on CT scanning on postoperative day 3 was managed with anticoagulation initially but was reexplored on day 9 due to hemorrhage and the graft was subsequently removed on day 11 due to severe hemorrhage and PVT. All other patients with grade 2 (n = 1) and 3 (n = 2) venous thrombosis were managed with anticoagulation and had a functioning graft at the end of study follow‐up.
3.4.3. Postoperative complications and length of hospital stay
Eight (33.3%) patients in the thrombosis group were reexplored for bleeding (3 preanticoagulation and 5 postanticoagulation patients), whereas 12 (15.2%) were reexplored for bleeding in the control (no thrombosis) group (P = .074). There were 21 early (within 30 days) complications in 13 patients in the thrombosis group and 39 complications in 31 patients in the control group (P = .241) (Table 5). The median length of postoperative stay (21 days vs 15 days; P = .001), the number of acute rejection episodes (8 [33.3%] vs 7 [8.9%]; P = .006), and the likelihood of CT finding of pancreatitis (12 [50.0%] vs 11 [13.9%]; P < .001) were significantly higher in the thrombosis group in comparison to the no‐thrombosis group (Table 5).
3.4.4. Risk factors for PAT
On multivariate analysis, PASPK/PAK transplant (odds ratio [OR] 1.09, confidence interval [CI] 1.01‐0.97, P = .047), acute rejection (OR 1.25, CI 1.07‐1.90, P = .034), and CT finding of pancreatitis (OR 1.23, CI 1.08‐1.72, P = .011) were risk factors for PAT (Table 6). The risk of vascular thrombosis was 9% higher with PASPK/PAK transplant in comparison to SPK transplant, 25% higher when there was acute rejection (although most cases of thrombosis were diagnoses early and before the diagnosis of rejection), and 23% higher when there were CT findings of allograft pancreatitis.
Table 6.
Risk factors for allograft thrombosis
| Thrombosis group (n = 24) | No‐thrombosis group (n = 79) | Multivariate analysisa | ||
|---|---|---|---|---|
| 95% CI | P value | |||
| Organ transplanted | ||||
| SPK | 18 (75.0%) | 78 (98.7%) | 0.01‐0.97 | .047 |
| PASPK/PAK | 4 (25.0%) | 1 (1.3%) | ||
| Acute rejection | 8 (33.3%) | 7 (8.9%) | 0.07‐0.90 | .034 |
| Peripancreatic edema and/or inflammatory changes on CT | 12 (50.0%) | 11 (13.9%) | 0.08‐0.72 | .011 |
The risk of vascular thrombosis was 9% higher with PASPK/PAK transplant in comparison to SPK transplant, 25% higher when there was acute rejection, and 23% higher when there were CT findings of allograft pancreatitis. P values that are significant are highlighted in bold.
CI, confidence interval; CT, computed tomography; OR, odds ratio; PAK, pancreas after kidney transplant; SPK, simultaneous pancreas–kidney transplant.
Logistic regression analysis.
3.4.5. Graft and patient survival
There were 6 (25.0%) graft losses in the thrombosis group with 3 grafts lost within 30 days due to portal vein thrombosis resulting in graft pancreatectomy on days 2, 5, and 11. The other 3 graft losses were due to severe pancreatitis with abscess formation in 1 patient and biopsy‐proved chronic rejection in 2 patients. There were 3 (3.8%) graft losses in the control group, 2 due to chronic rejection and 1 due to recurrence of “autoimmune” diabetes mellitus (Table 5). The 1‐, 3‐, and 5‐year death‐censored graft survival for thrombosis and no‐thrombosis groups were 75%, 75%, and 75% vs 100%, 90%, and 90%, respectively (log‐rank P < .0001) (Figure 2). There was no difference in patient survival between the 2 groups (1‐, 3‐, and 5‐year rates were 98%, 96%, and 94% in each group) (Figure 3). None of the patient deaths in the study groups were directly related to PAT (Table 5).
Figure 2.
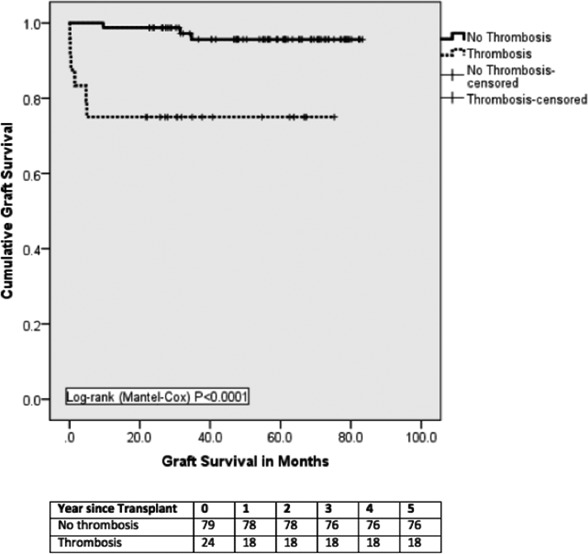
Death‐censored graft survival. Graft survival was significantly inferior in the thrombosis group compared to controls. All graft losses in this group occurred within two weeks of transplantation
Figure 3.
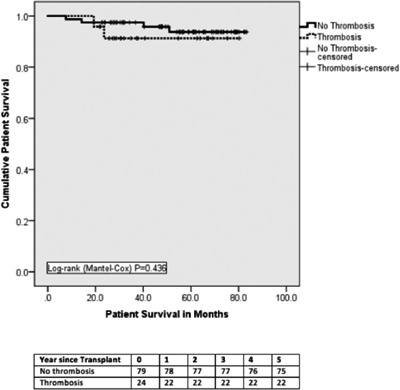
Patient survival. There was no difference in patient survival between thrombosis and no‐thrombosis groups
3.4.6. Risk factors for graft loss
On univariate analysis, recipient anastomosis time (48 ± 11 minutes in graft loss group vs 38 ± 9 minutes in control group; P = .001) and PAT (66.7% in graft loss group vs 19.1% in control group; P = .005) were statistically significant predictors of graft loss. On multivariate analysis, PAT was the only significant risk factor for graft loss (OR 1.17, 95% CI 1.04‐1.83, P = .028).
3.5. Independent retrospective review of CT scans
During the study period, 81 CT examinations were reported during the index admission. In total, 16 different senior radiologists reported the CT scans over the study period. On independent review of the CTs, previously unreported thromboses (additional thromboses) were identified by both review radiologists. In consensus, the review radiologists identified a total of 28 new thromboses: 17 grade 1 arterial, 2 grade 2 arterial, 5 grade 1 venous, and 4 grade 2 venous thromboses. No new grade 3 arterial or venous thromboses were identified on review (Table 7). Four patients who were initially reported to have thrombosis were subsequently reviewed as having no thrombosis. The thrombosis grade for these 4 patients on initial reports were grade 1 arterial, grade 3 arterial, and 2 patients with grade 1 venous.
Table 7.
Retrospective review of CT scans for allograft thrombosis
| Initial CT report | Radiologist 1 | Radiologist 2 | Consensus‐ Radiologists 1 and 2 | Consensus vs initial CT report (additional “new thrombosis”) | |
|---|---|---|---|---|---|
| No arterial or venous thrombosis | 58/81 | 37/81 | 33/81 | 28/81 | N/A |
| Arterial thrombosis | |||||
| Grade 0 (no thrombosis) | 66 | 48 | 44 | 38 | N/A |
| Grade 1 (peripheral) | 10 | 30 | 33 | 23 | 17 |
| Grade 2 (intermediate nonocclusive) | 4 | 3 | 4 | 3 | 2 |
| Grade 3 (central occlusive) | 1 | 0 | 0 | 0 | 0 |
| Venous thrombosis | |||||
| Grade 0 (no thrombosis) | 69 | 61 | 60 | 57 | N/A |
| Grade 1 (peripheral) | 5 | 9 | 11 | 7 | 5 |
| Grade 2 (intermediate nonocclusive) | 3 | 7 | 6 | 6 | 4 |
| Grade 3 (central occlusive) | 4 | 4 | 4 | 4 | 0 |
A total of 28 new thromboses were identified on retrospective review of the CT scans. Four patients who were initially reported to have thrombosis were subsequently reviewed as having no thrombosis.
Using the new retrospective diagnoses, we repeated the earlier analysis that is shown in Tables 1, 2, 3, 4 using the original diagnoses. Univariate analysis comparing those who had thrombosis on retrospective CT review (n = 53) with those who had no thrombosis (n = 28) demonstrated no significant predisposing factors for thrombosis (Tables S1‐S4).
3.6. Outcomes based on retrospective review of CT scans
Based on the retrospective review, patients were divided into 4 distinct groups according to the presence or absence of thrombosis (as a consensus of radiologists 1 and 2) and whether the recipients were anticoagulated. The retrospective review gave us an opportunity to compare outcomes in these groups, in a manner that would not be possible prospectively outside of a randomized controlled trial. Moreover, if we assume that the “new” thromboses diagnosed only on retrospective review were originally not reported at random, this approach reduces (but does not eliminate) bias.
Twenty‐six patients were identified to have thrombosis on retrospective CT review but were previously not anticoagulated (T‐NAC). Eighteen patients with thrombosis on retrospective review were anticoagulated (T‐AC). Compared with the T‐NAC group, T‐AC group demonstrated trends toward more reexploration for bleeding (7 [38.9%] vs 3 [11.5%]; P = .064), longer median length of stay (21 days vs 15 days; P = .077), and more peripancreatic edema and/or inflammatory changes on CT (10 [55.5%] vs 4 [15.4%]; P = .008). There were 2 grafts lost in the T‐AC group due to portal vein thrombosis (Table 8 and Figure 4).
Table 8.
Comparison of outcomes based on retrospective CT report and initial management
| T‐AC (all grades of thrombosis) (n = 18) | T‐NAC (n = 26) | T‐AC (grade 1 and 2 only) (n = 11) | NT‐AC (n = 6) | NT‐NAC (n = 31) | |
|---|---|---|---|---|---|
| Reexploration for bleeding |
7 (38.9%) [2 pre‐ and 5 post‐anticoagulation] P = .064 |
3 (11.5%) |
4 (36.4%) [2 pre‐ and 2 post‐anticoagulation] P = .210 |
3 (50.0%) [1 pre‐ and 2 post‐anticoagulation] |
5 (16.1%) |
| Length of postoperative stay (days) |
21 (10‐57) P = .077 |
15 (8‐305) |
17 (10‐38) P = .374 |
19 (17‐33) | 14 (6‐35) |
| Acute rejection |
5 (27.8%) P = .451 |
4 (15.4%) |
3 (27.3%) P = .638 |
3 (50.0%) | 2 (6.4%) |
| Peripancreatic edema and/or inflammatory changes on CT |
10 (55.5%) P = .008 |
4 (15.4%) |
7 (63.6%) P = .013 |
2 (33.3%) | 6 (19.3%) |
| Graft loss |
3 (16.7%) (PVT 2, chronic rejection) Log‐rank P = .123 |
1 (3.8%) (recurrence of DM) |
1 (9.1%) (chronic rejection) Log‐rank P = .567 |
2 (33.3%) (graft pancreatitis, chronic rejection) |
2 (6.4%) (chronic rejection, graft vasculopathy) |
| Patient death |
0 (0.0%) Log‐rank P = .281 |
2 (7.7%) (gastric and esophageal cancer) |
0 (0.0%) Log‐rank( )P = .353 |
1 (16.7%) | 1 (3.2%) |
A total of 28 new thromboses were identified on retrospective review of the CT scans. Four patients who were initially reported to have thrombosis were subsequently reviewed as having no thrombosis.
P values are comparison between 2 groups: Column 1: T‐AC (all grades of thrombosis) vs T‐NAC; Column 3: T‐AC (grade 1 and 2 thromboses only) vs T‐NAC. P values that are significant are highlighted in bold.
AC, anticoagulated; NAC, not anticoagulated; NT, no thrombosis; T, thrombosis.
Figure 4.
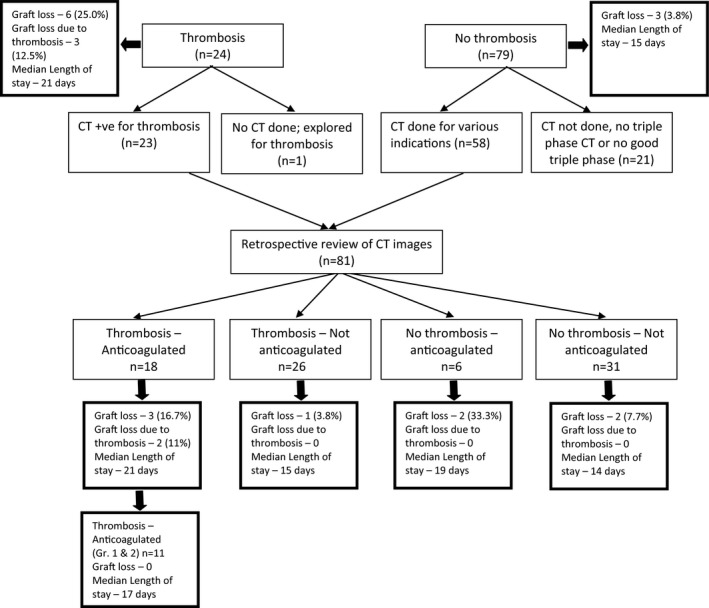
Flowchart depicting the 2 distinct stages of the retrospective study
When comparing the T‐NAC group (n = 26) with the T‐AC group with only grade 1 and 2 thromboses (n = 11), there was no difference in reexploration for bleeding (2 postanticoagulation [18.2%] vs 3 [11.5%]; P = .210) and median length of stay (17 days vs 15 days; P = .374). There was no graft loss due to thrombosis in those with T‐NAC and T‐AC with grade 1 and 2 thromboses only (Table 8). There was also no difference in graft and patient survival on comparing those who were anticoagulated for grade 1 and 2 thrombosis and those who were not anticoagulated (Figures 5 and 6).
Figure 5.
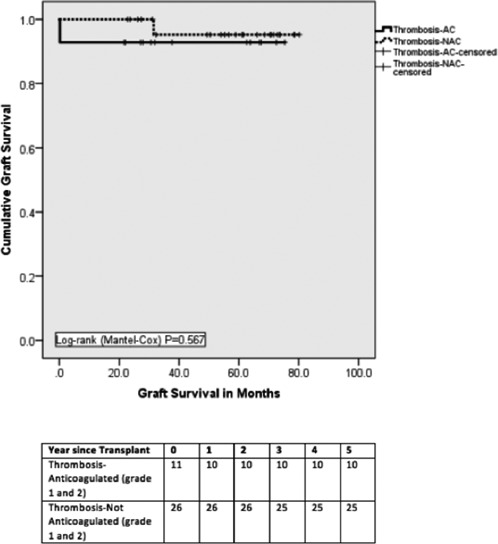
Death‐censored graft survival. Graft survival was similar between thrombosis‐Anticoagulated (Grade 1 and 2) and Thrombosis‐Not anticoagulated groups
Figure 6.
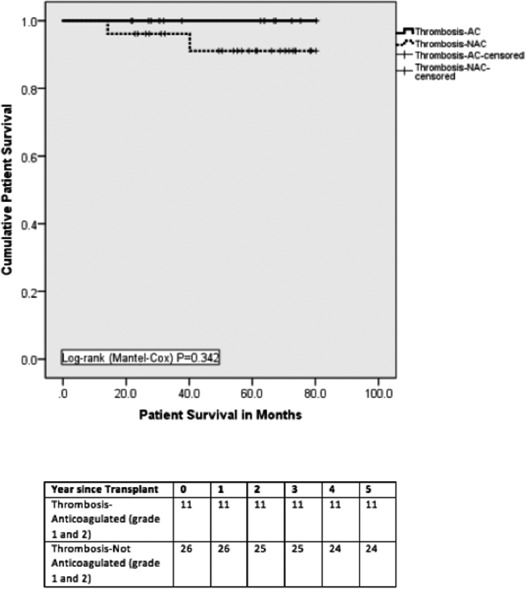
Patient survival. There was no statistical difference in patient survival between thrombosis‐Anticoagulated (Grade 1 and 2) and Thrombosis‐Not anticoagulated groups
Based on these findings, an algorithm for the management of thrombosis based on new CT grading system is presented in Figure 7.
Figure 7.
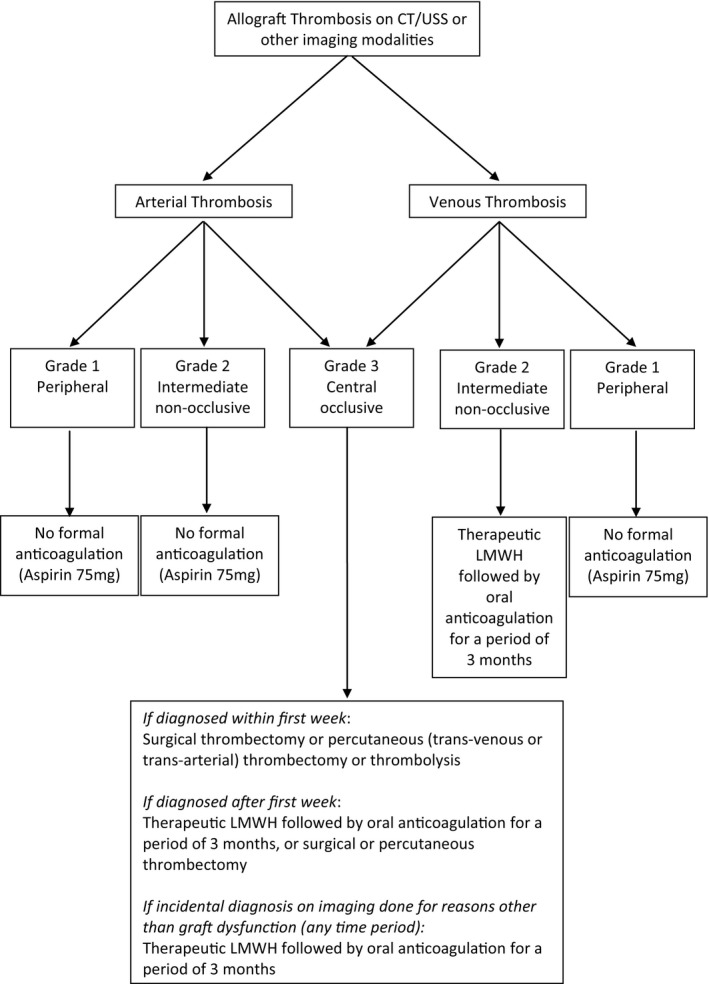
Management algorithm for allograft thrombosis based on the Cambridge Pancreas Allograft Thrombosis (CPAT) grading system
3.7. Evolution of thrombosis on retrospective review of CT scans
To determine if anticoagulation influences the evolution of grade 1 and 2 thromboses, we compared late CT images in the T‐AC and TNAC groups (Table 9). Retrospective review of CT images at 3 months of those who were anticoagulated for thrombosis demonstrated a reduction in grade of thrombosis in 5 of 8 patients and an increase in thrombosis grade in 2 patients. Review of last CT scans done at a median of 530 days posttransplant showed a decrease in thrombosis grade in 5 of 7 patients and an increase in grade in 2 patients. In those patients who were not anticoagulated for thrombosis, CT scans at 3 months showed 2 of 5 patients with reduction in grade of thrombosis and 1 patient with increase in thrombosis grade. Further CT scans at median of 670 days showed 10 of 15 patients with reduction in grade of thrombosis and 2 with increase in thrombosis grade.
Table 9.
Evolution of thrombus at 3 months and last CT scan
| Thrombosis anticoagulated (n = 18) | Thrombosis not anticoagulated (n = 26) | |
|---|---|---|
| First postoperative CT scan | Median 4 days | Median 4 days |
| Baseline CT vein scores | Grade 0 (n = 7) | Grade 0 (n = 17) |
| Grade 1 (n = 2) | Grade 1 (n = 7) | |
| Grade 2 (n = 5) | Grade 2 (n = 2) | |
| Grade 3 (n = 4) | Grade 3 (n = 0) | |
| Baseline CT artery scores | Grade 0 (n = 6) | Grade 0 (n = 5) |
| Grade 1 (n = 11) | Grade 1 (n = 19) | |
| Grade 2 (n = 1) | Grade 2 (n = 2) | |
| Grade 3 (n = 0) | Grade 3 (n = 0) | |
| Peripancreatic edema and/or peripancreatic inflammatory changes at baseline CT scan | 13 | 16 |
| CT scan 3 months | Median 117 days (8 patients) | Median 96 days (5 patients) |
| CT vein at 3 monthsa | Previous thrombosis downgraded (n = 3) [3 to 2, 2 to 0, and 1 to 0] | Previous thrombosis downgraded (n = 1) [1 to 0] |
| CT artery at 3 monthsa | Previous thrombosis downgraded (n = 2) [both 1 to 0] | Previous thrombosis downgraded (n = 1) [1 to 0] |
| Previous thrombosis upgraded (n = 2) [both from 1 to 2] | Previous thrombosis upgraded (n = 1) [0 to 2] | |
| Peripancreatic edema at 3‐month CT | 1 | 2 |
| Last CT scan | Median 530 days (7 patients) | Median 670 days (15 patients) |
| Last CT veina | Previous thrombosis downgraded (n = 2) [2 to 0 and 1 to 0] | Previous thrombosis downgraded (n = 4) [all 1 to 0] |
| Last CT arterya | Previous thrombosis downgraded (n = 3) [all 1 to 0] | Previous thrombosis downgraded (n = 6) [1 to 0] |
| Previous thrombosis upgraded (n = 2) [0 to 3, 1 to 3] | Previous thrombosis upgraded (n = 2) [0 to 2, 1 to 2] | |
| Peripancreatic edema on last CT | 2 | 0 |
The evolution of thrombosis was not obviously affected by anticoagulation.
Comparison of 3‐month CT and last follow‐up CT was made with the first postoperative CT scan.
4. DISCUSSION
The incidence of PAT in our center is 23.3% (n = 24). The rate is high because we have included grade 1 thrombosis (peripheral thrombus), which forms a majority of our thromboses (45.8%; n = 11). We believe many of these are not reported in previous publications. Only 5.8% of recipients in our series were diagnosed with occlusive (grade 3) thrombosis. Moreover, the incidence of graft loss secondary to thrombosis was only 2.9%.5, 28 While managements of recipients with PAT must be primarily based on individual clinical circumstances, we believe that our analysis can potentially inform and enhance evidence‐based clinical decision‐making. Based on our proposed grading system, henceforth termed the Cambridge Pancreas Allograft Thrombosis (CPAT) grading, our data suggest that grade 1 and 2 arterial thromboses and grade 1 venous thrombosis can be managed safely without formal anticoagulation. Although we report 17% higher risk of graft loss with thrombosis, all of the graft losses in our cohort were due to grade 2 or 3 thrombosis and grade 1 thrombosis had no effect on graft or patient survival. The risk factors for PAT in our cohort were pancreas transplantation into a nonuremic patient, acute rejection, and pancreatitis on CT, similar to previous published literature.29, 30
The important findings from our study are that arterial thrombosis presented late (median 5 days) compared with venous thrombosis (median 3 days) and most cases of arterial thrombosis could be managed with anticoagulation alone. In comparison, more than half of our cases of venous thromboses were diagnosed within the first 72 hours and prompted reexploration; there was higher risk of graft loss when a grade 3 thrombosis was present. Arterial thrombosis including both partial (grades 1 and 2) and complete (grade 3) were managed with anticoagulation alone in our cohort with no graft loss, albeit the grade 3 thrombosis on retrospective review was reported as no thrombosis. Only 1 graft was reexplored for grade 1 arterial thrombosis (thrombus distally in the graft), but there was also concern about extrinsic compression of the portal vein on this patient's CT scan, which contributed significantly to the decision to reexplore. At reexploration, there was only evidence of severe graft pancreatitis and no arterial thrombosis. This was the only negative laparotomy in our study cohort. On retrospective review of this patient's CT scan, radiologist 1 reported no arterial thrombosis and radiologist 2 reported a grade 1 arterial thrombosis. Fridell et al, in a retrospective review of 35 reexplorations for thrombosis in 345 transplantations over a 7‐year period, reported a negative laparotomy rate of 43%.18
The proposed CPAT grading system helps clinicians make decisions on which grafts may require reexploration and which can be managed with anticoagulation alone. The retrospective review of the CT scans allowed us to compare 2 groups – one therapeutically anticoagulated for grade 1 and 2 thromboses and one managed conservatively. Because the diagnoses of thrombosis in the nonanticoagulated group were not reported at random, this reduces potential bias arising from allocation of patients to the 2 groups. In this study, therefore, patients were in effect “inadvertently randomized” into anticoagulation and conservative groups at the time of the index admission. Importantly, we were able to compare outcomes in the 2 groups where the CPAT grades of the thrombosis were comparable (grades 1 and 2). Those who were managed without anticoagulation had similar graft and patient survivals. Of note is that the morbidities associated with anticoagulation such as reexploration for bleeding were numerically less in the nonanticoagulated group (11.5% vs 36.4%; P = .201) and, hence, the length of stay was shorter (15 days vs 17 days; P = .374). An ideal prospective study would be to randomize patients with grade 1 and 2 (partial thrombosis) into anticoagulation vs no‐anticoagulation arms. Such a study would need large number of transplants to adequately power the study and may not have clinical equipoise to justify such randomization. Although the CPAT grading system has been used to report CT studies, we believe the same grading system could be applicable for USS or CEUS or MR reporting of PAT.
Management of PAT without anticoagulation (except aspirin) has been reported previously with no deleterious effects on the graft. Ciancio et al. reported 6 patients with partial PAT of the splenic vein, who were started on aspirin 81 mg once daily and followed with serial Doppler USS. None progressed to complete thrombosis, and all had a functioning graft at the end of follow‐up.31 Delis et al. managed partial venous thrombosis (n = 10) with aspirin alone and showed no progression of thrombosis. They reported recanalization of the thrombosed veins on follow‐up imaging and, overall, there was no effect on graft survival.32 Our retrospective review of images at 3 months and subsequently showed that majority of patients who were not anticoagulated for thrombosis had a reduction in grade of thrombosis to no thrombosis or lower grade thrombosis. This is in keeping with our suggestion that formal anticoagulation with LMWH is unnecessary in grade 1 and 2 arterial and grade 1 venous thromboses. Thromboelastography‐directed anticoagulation may have a role in identifying patients who need anticoagulation in the postoperative period.33
The major strength of our study is the inclusion of unselected, consecutive patients from a single center. The retrospective nature of the study and the relatively small number of patients are obvious limitations. There were no changes in the preoperative assessment of the recipients, immunosuppression protocols, or postoperative management during the study period, thereby reducing the bias associated with the retrospective studies. Triple‐phase CT was requested in the post‐operative period to rule out thrombosis if there was a rise in pancreatic enzyme levels (amylase or lipase) or raised glucose (>10 mmol/L). Hence, these scans were not driven by any routine protocol. However, it is difficult to ascertain if those who did not have CT (one‐fifth of our patients) may have had subclinical thrombosis, and therefore our results may not give a true picture of the incidence of thrombosis. It is also likely that too many CT scans could have picked up peripheral thrombosis (grade 1), which would not have altered clinical outcome but resulted in therapeutic anticoagulation in our cohort. The retrospective independent and blinded review of CT examinations by 2 radiologists gave us a specific cohort, allowing comparison of those who had grade 1 and 2 thromboses and were managed with and without anticoagulation. Although retrospective combined review of CT examinations resulted in a greater number of small‐volume thromboses being identified, those except central occlusive thromboses in either the arterial or venous system can be managed without anticoagulation.
5. CONCLUSION
This retrospective study reports findings that suggest thromboses that do not involve the central vessels (portal vein or Y‐graft) of pancreatic grafts (grade 1 or grade 2) can be managed without anticoagulation. The proposed CPAT grading system may help reporting of thrombosis, provide prognostic information for the individual patient, and allow better comparison of reports from different centers for improved understanding and management of this common condition. Future studies should focus on reporting outcomes when partial thrombosis is managed without anticoagulation. Prospective studies are needed to verify the suggested protocol, and ascertain the evolution of allograft thrombosis when the patients are anticoagulated, and to determine the optimal duration of anticoagulation.
AUTHOR CONTRIBUTION
KSP and AH planned and designed the study. JC and SI collected the clinical data needed for the study. AH performed the statistical analysis and wrote the manuscript. All coauthors reviewed and revised the study design and the manuscript, which was finalized by KSP. SU and EMG defined the grading system used and carried out the retrospective review of CT images.
DISCLOSURE
The authors of this manuscript have no conflicts of interests to disclose as described by the American Journal of Transplantation.
Supporting information
ACKNOWLEDGMENTS
The research was supported by the National Institute for Health Research Blood and Transplant Research Unit (NIHR BTRU) in Organ Donation and Transplantation at the University of Cambridge in collaboration with Newcastle University and in partnership with NHS Blood and Transplant (NHSBT). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health, or NHSBT.
Hakeem A, Chen J, Iype S, et al. Pancreatic allograft thrombosis: Suggestion for a CT grading system and management algorithm. Am J Transplant. 2018;18:163–179. https://doi.org/10.1111/ajt.14433
REFERENCES
- 1. Farney AC, Rogers J, Stratta RJ. Pancreas graft thrombosis: causes, prevention, diagnosis, and intervention. Curr Opin Organ Transplant. 2012;17(1):87‐92. [DOI] [PubMed] [Google Scholar]
- 2. Ramessur Chandran S, Kanellis J, Polkinghorne KR, Saunder AC, Mulley WR. Early pancreas allograft thrombosis. Clin Transplant. 2013;27(3):410‐416. [DOI] [PubMed] [Google Scholar]
- 3. Muthusamy AS, Giangrande PL, Friend PJ. Pancreas allograft thrombosis. Transplantation. 2010;90(7):705‐707. [DOI] [PubMed] [Google Scholar]
- 4. Burke GW 3rd, Ciancio G, Figueiro J, et al. Hypercoagulable state associated with kidney‐pancreas transplantation. Thromboelastogram‐directed anti‐coagulation and implications for future therapy. Clin Transplant. 2004;18(4):423‐428. [DOI] [PubMed] [Google Scholar]
- 5. Shahrestani S, Webster AC, Lam VW, et al. Outcomes from pancreatic transplantation in donation after cardiac death: a systematic review and meta‐analysis. Transplantation. 2017;101(1):122‐130. [DOI] [PubMed] [Google Scholar]
- 6. Salvalaggio PR, Davies DB, Fernandez LA, Kaufman DB. Outcomes of pancreas transplantation in the United States using cardiac‐death donors. Am J Transplant. 2006;6(5 Pt 1):1059‐1065. [DOI] [PubMed] [Google Scholar]
- 7. Patel SR, Hakim N. Prevention and management of graft thrombosis in pancreatic transplant. Exp Clin Transplant. 2012;10(3):282‐289. [DOI] [PubMed] [Google Scholar]
- 8. Gruessner AC, Gruessner RW. Pancreas Transplantation of US and Non‐US Cases from 2005 to 2014 as Reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR). Rev Diabet Stud. 2016;13:35‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bozlar U, Brayman KL, Hagspiel KD. Pancreas allografts: comparison of three‐dimensional rotational angiography with standard digital subtraction angiography. J Vasc Interv Radiol. 2008;19(2 Pt 1):239‐244. [DOI] [PubMed] [Google Scholar]
- 10. O'Malley RB, Moshiri M, Osman S, Menias CO, Katz DS. Imaging of pancreas transplantation and its complications. Radiol Clin North Am. 2016;54(2):251‐266. [DOI] [PubMed] [Google Scholar]
- 11. Tolat PP, Foley WD, Johnson C, Hohenwalter MD, Quiroz FA. Pancreas transplant imaging: how I do it. Radiology. 2015;275(1):14‐27. [DOI] [PubMed] [Google Scholar]
- 12. Yates A, Parry C, Stephens M, Eynon A. Imaging pancreas transplants. Br J Radiol. 1030;2013(86):20130428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liong SY, Dixon RE, Chalmers N, Tavakoli A, Augustine T, O'Shea S. Complications following pancreatic transplantations: imaging features. Abdom Imaging. 2011;36(2):206‐214. [DOI] [PubMed] [Google Scholar]
- 14. Vandermeer FQ, Manning MA, Frazier AA, Wong‐You‐Cheong JJ. Imaging of whole‐organ pancreas transplants. Radiographics. 2012;32(2):411‐435. [DOI] [PubMed] [Google Scholar]
- 15. Kim YH, Park JB, Lee SS, Byun JH, Kim SC, Han DJ. How to avoid graft thrombosis requiring graftectomy: immediate posttransplant CT angiography in pancreas transplantation. Transplantation. 2012;94(9):925‐930. [DOI] [PubMed] [Google Scholar]
- 16. Siskind E, Maloney C, Ashburn S, et al. The use of venous jump grafts in pancreatic transplantation ‐ no difference in patient or allograft outcomes ‐ an update of the UNOS database. Clin Transplant. 2014;28(8):883‐888. [DOI] [PubMed] [Google Scholar]
- 17. Barrufet M, Burrel M, Angeles Garcia‐Criado M, et al. Pancreas transplants venous graft thrombosis: endovascular thrombolysis for graft rescue. Cardiovasc Intervent Radiol. 2014;37(5):1226‐1234. [DOI] [PubMed] [Google Scholar]
- 18. Fridell JA, Mangus RS, Mull AB, et al. Early reexploration for suspected thrombosis after pancreas transplantation. Transplantation. 2011;91(8):902‐907. [DOI] [PubMed] [Google Scholar]
- 19. Han K, Ko HK, Tsauo J, et al. Endovascular management for the treatment of pancreas transplant venous thrombosis: a single‐center experience. J Vasc Interv Radiol. 2016;27(6):882‐888. [DOI] [PubMed] [Google Scholar]
- 20. Saad WE, Darwish WE, Turba UC, et al. Endovascular management of vascular complications in pancreatic transplants. Vasc Endovascular Surg. 2012;46(3):262‐268. [DOI] [PubMed] [Google Scholar]
- 21. Margreiter C, Mark W, Wiedemann D, et al. Pancreatic graft survival despite partial vascular graft thrombosis due to splenocephalic anastomoses. Am J Transplant. 2010;10(4):846‐851. [DOI] [PubMed] [Google Scholar]
- 22. Maraschio MA, Kayler LK, Merion RM, et al. Successful surgical salvage of partial pancreatic allograft thrombosis. Transpl Proc. 2003;35(4):1491‐1493. [DOI] [PubMed] [Google Scholar]
- 23. Matsumoto I, Shinzeki M, Asari S, et al. Functioning pancreas graft with thromboses of splenic and superior mesenteric arteries after simultaneous pancreas‐kidney transplantation: a case report. Transpl Proc. 2014;46(3):989‐991. [DOI] [PubMed] [Google Scholar]
- 24. Choi BH, Lee HY, Park YM, Yang KH, Ryu JH, Chu CW. Formation of collateral veins in a graft pancreas after a simultaneous pancreas and kidney transplantation: a case report. Transpl Proc. 2015;47(7):2270‐2273. [DOI] [PubMed] [Google Scholar]
- 25. Scheffert JL, Taber DJ, Pilch NA, Chavin KD, Baliga PK, Bratton CF. Clinical outcomes associated with the early postoperative use of heparin in pancreas transplantation. Transplantation. 2014;97(6):681‐685. [DOI] [PubMed] [Google Scholar]
- 26. Schenker P, Vonend O, Ertas N, et al. Incidence of pancreas graft thrombosis using low‐molecular‐weight heparin. Clin Transplant. 2009;23(3):407‐414. [DOI] [PubMed] [Google Scholar]
- 27. Aboalsamh G, Anderson P, Al‐Abbassi A, McAlister V, Luke PP, Sener A. Heparin infusion in simultaneous pancreas and kidney transplantation reduces graft thrombosis and improves graft survival. Clin Transplant. 2016;30:1002‐1009. [DOI] [PubMed] [Google Scholar]
- 28. Nagai S, Powelson JA, Taber TE, Goble ML, Mangus RS, Fridell JA. Allograft pancreatectomy: indications and outcomes. Am J Transplant. 2015;15(9):2456‐2464. [DOI] [PubMed] [Google Scholar]
- 29. Gruessner AC. 2011 update on pancreas transplantation: comprehensive trend analysis of 25,000 cases followed up over the course of twenty‐four years at the International Pancreas Transplant Registry (IPTR). Rev Diabet Stud. 2011;8(1):6‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gruessner AC, Gruessner RW. Long‐term outcome after pancreas transplantation: a registry analysis. Curr Opin Organ Transplant. 2016;21:377‐385. [DOI] [PubMed] [Google Scholar]
- 31. Ciancio G, Cespedes M, Olson L, Miller J, Burke GW. Partial venous thrombosis of the pancreatic allografts after simultaneous pancreas‐kidney transplantation. Clin Transplant. 2000;14(5):464‐471. [DOI] [PubMed] [Google Scholar]
- 32. Delis S, Dervenis C, Bramis J, Burke GW, Miller J, Ciancio G. Vascular complications of pancreas transplantation. Pancreas. 2004;28(4):413‐420. [DOI] [PubMed] [Google Scholar]
- 33. Vaidya A, Muthusamy AS, Hadjianastassiou VG, et al. Simultaneous pancreas–kidney transplantation: to anticoagulate or not? Is that a question? Clin Transplant. 2007;21(4):554‐557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


