Abstract
Aims
Insulin glargine 300 U/mL (Gla‐300) offers a flatter pharmacodynamic profile than insulin glargine 100 U/mL (Gla‐100). We have compared these insulins over 1 year in people with type 1 diabetes (T1DM).
Methods
EDITION 4 was a 6‐month, multicentre, randomized, open‐label phase 3 study. People with T1DM who completed the 6 months continued randomized Gla‐300 or Gla‐100 once daily, morning or evening, for a further 6 months.
Results
Among 549 participants randomized, 444 completed the 12‐month study period (Gla‐300, 80%; Gla‐100, 82%). Mean HbA1c decreased similarly from baseline to month 12 in the 2 treatment groups (difference, 0.02 [95% CI, −0.13 to 0.17]) %‐units [0.2 (−1.5 to 1.9) mmol/mol]), to a mean of 7.86 %‐units (62.4 mmol/mol) in both groups. For morning vs evening injection, there was no difference in HbA1c change over 12 months for Gla‐100, but a significantly larger decrease in HbA1c was observed in the Gla‐300 morning group than in the Gla‐300 evening group (difference, −0.25 [−0.47 to −0.04] %‐units [−2.7 (−5.2 to −0.4) mmol/mol]). Mean glucose from the 8‐point SMPG profiles decreased from baseline, and was similar between the 2 treatment groups. Basal insulin dose was 20% higher with Gla‐300 than with Gla‐100, while hypoglycaemia event rates, analysed at night, over 24 hours, or according to different glycaemic thresholds, did not differ between treatment groups, regardless of injection time. Adverse event profiles did not differ between groups.
Conclusions
In T1DM, Gla‐300 provides glucose control comparable to that of Gla‐100, and can be given at any time of day.
Keywords: analogues, basal insulin, clinical trial, glycaemic control, hypoglycaemia, insulin, type 1 diabetes
1. INTRODUCTION
In people with type 1 diabetes (T1DM), long‐term maintenance of blood glucose levels close to normal is associated with a reduction in progression of microvascular complications and in all‐cause mortality.1, 2 Current guidelines recommend insulin analogues for people with T1DM, because of the lower associated risk of hypoglycaemia compared with optimized insulin therapy with human insulin.3 Nevertheless, blood glucose control remains suboptimal in many people,4, 5 with described barriers including concerns about hypoglycaemia, flexibility of injection schedule and weight gain.5 Some of these difficulties may be related to action profiles of conventional extended‐acting insulins and the first‐generation long‐acting insulin analogues.5, 6
In people with type 2 diabetes (T2DM) already receiving insulin, the more stable and prolonged pharmacokinetic and pharmacodynamic profiles of insulin glargine 300 U/mL (Gla‐300) vs those of glargine 100 U/mL (Gla‐100)7 translate into sustained glycaemic control for 1 year, with less nocturnal hypoglycaemia.8, 9 In T1DM in the EDITION 4 clinical trial, 549 people were randomized to Gla‐300 or Gla‐100 as basal insulin, and to morning or evening injection.10 The primary 6‐month results showed equivalent glycaemic control and a lower risk of nocturnal hypoglycaemia with Gla‐300 vs Gla‐100 during the first 8 weeks.10 Glucose profiles, rates of hypoglycaemia and adverse events were comparable, irrespective of morning or evening Gla‐300 injection,10 suggesting that Gla‐300 provides the freedom to choose a morning or evening injection schedule without compromising glycaemic control or increasing hypoglycaemia. In the current 6‐month, pre‐planned continuation of EDITION 4, we investigated the safety, tolerability and efficacy of Gla‐300 compared with Gla‐100, given morning or evening, over 12 months.
2. MATERIALS AND METHODS
2.1. Study design and participants
EDITION 4 was a multicentre, 4‐arm, parallel‐group, phase 3a study in people with T1DM who were randomized (1:1:1:1) to once‐daily Gla‐300 or Gla‐100 (both Sanofi, Paris, France), injected morning or evening, while continuing meal‐time insulin (NCT01683266).10 People completing the 6‐month main study period continued open‐label Gla‐300 or Gla‐100 once daily in the morning or evening, as previously randomized, for a further 6 months. The EDITION 4 study design was reported in detail in the primary 6‐month report.10
Participants were aged ≥18 years, had T1DM for >1 year, had HbA1c in the range of 7.0 %‐units to 10.0 %‐units (53 to 86 mmol/mol) and had spent >1 year on basal insulin combined with a meal‐time insulin analogue. Gla‐300 was given subcutaneously using a modified TactiPen pen‐injector (Sanofi) that allowed 1.5 U dose increments, while Gla‐100 was given subcutaneously using the SoloSTAR pen (Sanofi), allowing 1 U dose increments.
Basal insulin was titrated to a pre‐breakfast self‐monitored plasma glucose (SMPG) level of 4.4 to 7.2 mmol/L (80–130 mg/dL).10 Dose adjustments of basal insulin were made weekly, and no more often than every 3 to 4 days.10 Meal‐time insulin was continued with a target range of <8.9 mmol/L (160 mg/dL) for 2‐hour postprandial plasma glucose, adjusted at investigator/participant discretion.10
The study was approved by relevant review boards/ethics committees, and was performed in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines. All participants provided written informed consent.10
2.2. Outcomes
Efficacy outcomes included change from baseline to month 12 in HbA1c, central‐laboratory‐measured fasting plasma glucose (FPG), pre‐breakfast SMPG, 8‐point SMPG profiles and insulin dose (basal and meal‐time).
Hypoglycaemia assessments included the percentage of participants reporting ≥1 event and event rates. Events were categorized using American Diabetes Association (ADA) definitions11: “severe” hypoglycaemia was defined as an event that required assistance; “documented symptomatic” hypoglycaemia required typical symptoms with a plasma glucose concentration ≤3.9 mmol/L (≤70 mg/dL); “confirmed or severe” hypoglycaemia included symptomatic or asymptomatic events with plasma glucose concentration ≤3.9 mmol/L (≤70 mg/dL) and “severe” events. A pre‐planned sensitivity analysis using <3.0 mmol/L (<54 mg/dL) was done. “Night‐time” was defined as 0:00 to 5:59 am.
Treatment‐emergent adverse events (AEs) and body weight were systematically recorded at each visit. Participant‐reported satisfaction with treatment and perception of occurrence of hypo‐ and hyper‐glycaemia were assessed using the Diabetes Treatment Satisfaction Questionnaire (status) (DTSQs), and health‐related quality of life was assessed with the EQ‐5D questionnaire.12, 13 Behaviours and worries related to hypoglycaemia were assessed with the Hypoglycaemia Fear Survey‐II (HFS II).14
2.3. Data analysis and statistics
Efficacy analyses were based on the modified intention‐to‐treat (mITT) population, defined as all randomized participants who received ≥1 dose of study insulin and had a baseline and at least one post‐baseline efficacy assessment. Continuous endpoints were analysed using a mixed model for repeated measures (MMRM) approach, and categorical variables were analysed using a Cochran–Mantel–Haenszel (CMH) method.10
Hypoglycaemia and safety analyses were based on the safety population: all participants randomized and receiving ≥1 treatment dose. Change in body weight was assessed using an analysis of covariance (ANCOVA) model. Calculation of rate ratio for hypoglycaemic event rate was done using an over‐dispersed Poisson regression model. AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 17.0.
3. RESULTS
3.1. Participants
All 549 people with T1DM who were randomized to Gla‐300 (n = 274) or Gla‐100 (n = 275) received study insulin (the safety population) (Figure S1). All participants randomized to Gla‐300 met criteria for the mITT population (above), but there was no baseline or post‐baseline efficacy data for 2 Gla‐100 participants (n = 273 mITT participants). In the Gla‐300 group, 136 and 138 participants received morning and evening injections, respectively, and in the Gla‐100 group, 135 and 138 participants received morning and evening injections, respectively.
The 12‐month study period was completed by 219 participants (80%) in the Gla‐300 group and by 225 (82%) in the Gla‐100 group (Figure S1). Most discontinuations occurred in the first 6 months of the study (Gla‐300, 43; Gla‐100, 39), with few during the second 6 months (Gla‐300, 12; Gla‐100, 11). The most common explanation given for treatment discontinuation was “other reasons” (32 [12%] and 38 [14%] participants in the 2 groups, respectively), usually stating personal/family or job‐conflict reasons (17 and 25 participants, respectively).
Baseline characteristics have been fully described previously.10 Mean (SD) age of participants was 47.3 (13.7) years; 57% were male; BMI was 27.6 (5.1) kg/m2; and body weight was 81.8 (18.7) kg. Duration of T1DM was 21.0 (12.9) years and most participants (82%) transferred from insulin glargine. Baseline HbA1c was 8.13 (0.8) %‐units (65.3 [9] mmol/mol) and laboratory‐measured clinic FPG was 10.7 (4.4) mmol/L (193 [79] mg/dL). No notable differences in baseline characteristics were observed between the insulin groups or in morning vs evening injection.
3.2. Insulin dose
Mean (SD) daily basal insulin dose prior to the study was 0.38 (0.17) U/kg (32.3 [20.8] U) in the Gla‐300 group and 0.37 (0.15) U/kg (30.6 [15.2] U) in the Gla‐100 group. These doses were lower at day 1 (“baseline”) (Table S1) and then increased to month 12 in both groups (Gla‐300 to 0.48 [0.22] U/kg; Gla‐100 to 0.40 [0.18] U/kg), mostly during the first 8 weeks (Figure 1A). The meal‐time total insulin dose remained stable in both groups throughout the study.
Figure 1.
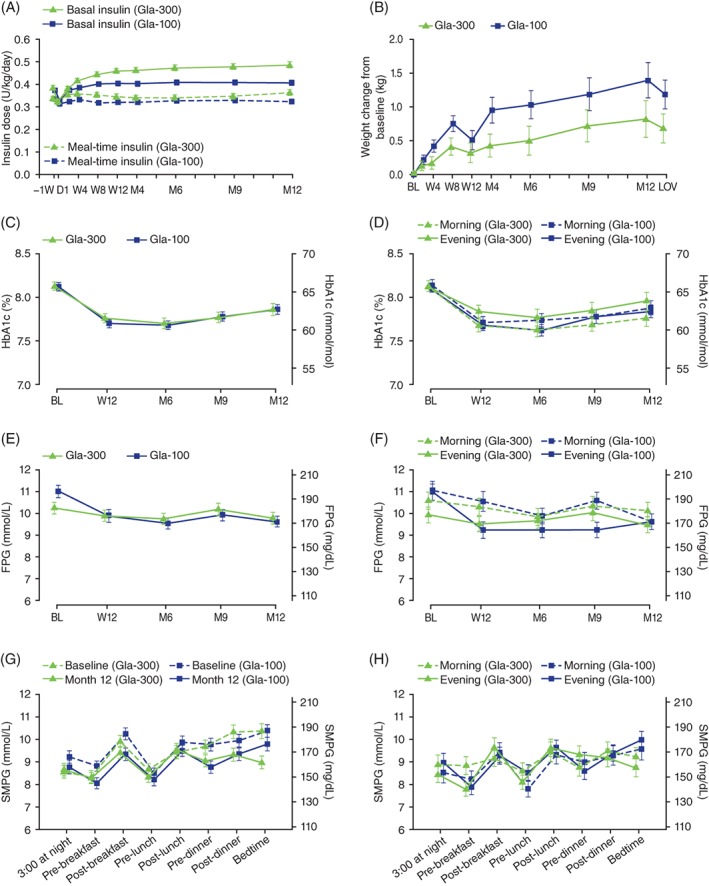
Time course of daily insulin dose (A), body weight change from baseline (B), glycated haemoglobin (C), glycated haemoglobin by basal insulin injection time (D), laboratory‐measured clinic FPG (E), laboratory‐measured clinic FPG by basal insulin injection time (F), SMPG profiles (G) and SMPG profiles by basal insulin injection time (H) over 12 months of treatment with Gla‐300 or Gla‐100. mITT population, except body weight change (safety population). Baseline body weight = 81.9 and 81.8 kg for Gla‐300 and Gla‐100 groups, respectively. Mean ± SE. BL, baseline; D, day; FPG, laboratory‐measured clinic fasting plasma glucose; LOV, last on‐treatment value; M, month; mITT, modified intention‐to‐treat; SE, standard error; SMPG, self‐monitored plasma glucose; W, week
Mean daily basal insulin doses at 12 months in the Gla‐300 group were 0.51 (0.23) U/kg for morning injections and 0.46 (0.21) U/kg for evening injections; corresponding doses in the Gla‐100 group were 0.45 (0.18) U/kg and 0.36 (0.17) U/kg (Table S1). Total daily insulin dose at month 12 for morning injections was 15.6% higher in the Gla‐300 group (0.87 [0.33] U/kg) than in the Gla‐100 group (0.75 [0.25] U/kg), and for evening injections it was 16.8% higher (0.82 [0.36] vs 0.70 [0.28] U/kg). At month 12, while basal insulin was 58.2% of total dose with Gla‐300 and, similarly, 59.5% with Gla‐100 in the morning injection groups, these proportions were 56.1% and 51.7% in the evening groups.
3.3. Blood glucose control
Mean HbA1c decreased similarly from baseline to month 12 in the Gla‐300 and Gla‐100 groups (Figure 1C and Table 1). LS mean difference in change from baseline (Gla‐300 vs Gla‐100) was 0.02 (95% CI, −0.13 to 0.17) %‐units (0.2 [95% CI,−1.5 to 1.9] mmol/mol), with most of the decrease by week 12. During the second 6‐month period, mean HbA1c increased similarly in the 2 groups, remaining below baseline and ending at 7.86 (SD, 1.03) %‐units (62.4 [SD, 11.3] mmol/mol) and 7.86 (SD, 0.84) %‐units (62.4 [SD, 9.2] mmol/mol) in the Gla‐300 and Gla‐100 groups, respectively (Figure 1C). When comparing morning and evening injection (Figure 1D), there was no difference in HbA1c change over 12 months for the Gla‐100 group, but a significantly larger decrease was observed in the Gla‐300 morning group (ending at 7.76 [SD, 0.98] %‐units [61.4 (SD, 10.7) mmol/mol]) than in the Gla‐300 evening group (ending at 7.96 [SD, 1.07] %‐units [63.5 (SD, 11.7) mmol/mol]) (LS mean difference [95% CI] in change from baseline, −0.25 [−0.47 to −0.04] %‐units (−2.7 [−5.2 to −0.4] mmol/mol).
Table 1.
Glycaemic control measures over 12 months of treatment with Gla‐300 or Gla‐100
| Gla‐300 | Gla‐100 | |
|---|---|---|
| HbA1c (%) | ||
| Baseline | 8.13 (0.77) | 8.12 (0.79) |
| Month 12 | 7.86 (1.03) | 7.86 (0.84) |
| Change | −0.20 (0.06) | −0.22 (0.06) |
| LS mean difference (95% CI) | 0.02 (−0.13 to 0.17) | |
| HbA1c (mmol/mol) | ||
| Baseline | 65.3 (8.4) | 65.2 (8.6) |
| Month 12 | 62.4 (11.3) | 62.4 (9.2) |
| Change | −2.2 (0.6) | −2.4 (0.6) |
| LS mean difference (95% CI) | 0.2 (−1.5 to 1.9) | |
| FPG (mmol/L) | ||
| Baseline | 10.26 (4.14) | 11.02 (4.46) |
| Month 12 | 9.79 (3.87) | 9.63 (3.62) |
| Change | −0.43 (5.22) | −1.39 (5.43) |
| LS mean difference (95% CI) | 0.18 (−0.55 to 0.90) | |
| Average 24‐h SMPG (mmol/L) | ||
| Baseline | 9.40 (2.54) | 9.60 (2.34) |
| Month 12 | 9.02 (2.23) | 8.99 (2.22) |
| Change | −0.36 (2.77) | −0.58 (2.87) |
| LS mean difference (95% CI) | 0.12 (−0.34 to 0.58) | |
| Pre‐breakfast SMPG (mmol/L) | ||
| Baseline | 8.91 (2.56) | 9.28 (2.51) |
| Month 12 | 8.44 (2.29) | 8.40 (2.09) |
| Change | −0.32 (2.98) | −0.76 (2.71) |
Abbreviations: FPG, laboratory‐measured clinic fasting plasma glucose; mITT, modified intention‐to‐treat; SD, standard deviation; SMPG, self‐monitored plasma glucose.
mITT population. Data are presented as mean (SD) unless otherwise mentioned.
A comparable reduction in laboratory‐measured clinic FPG from baseline to month 12 was seen with Gla‐300 and Gla‐100 (Figure 1E and Table 1). LS mean difference in change from baseline to month 12 was 0.18 (95% CI, −0.55 to 0.90) mmol/L (3.2 [−10.0 to 16.3] mg/dL). No effect of injection time was observed for the time course of laboratory‐measured FPG, with comparable reductions by month 12 for evening and morning groups (Figure 1F).
By month 12, plasma glucose levels on 8‐point SMPG profiles had decreased at all time points compared with baseline, and were comparable between the insulin groups, except at bedtime, with lower levels in the Gla‐300 group than in the Gla‐100 group (Figure 1G). At month 12, 8‐point SMPG profiles were comparable between morning and evening injection groups for Gla‐300 and Gla‐100 (Figure 1H), except pre‐breakfast, which was lower for evening injections than for morning injections with Gla‐300.
3.4. Hypoglycaemia
Over 12 months nearly all participants (260 [~95%] in each group) experienced ≥1 confirmed (≤3.9 mmol/L [≤70 mg/dL]) or severe hypoglycaemic event (risk ratio, 1.00 [0.96–1.05]); most participants (199 [73%] for Gla‐300 and 205 [75%] for Gla‐100) experienced ≥1 nocturnal event (risk ratio, 0.97 [0.88–1.08]) (Table 2). Annualized rates of confirmed or severe hypoglycaemia were 75.9 events/person‐year in the Gla‐300 group and 68.8 events/person‐year in the Gla‐100 group at any time of day (24 hours) (rate ratio, 1.11 [0.97–1.29]), and 8.1 and 8.6 events/person‐year at night (rate ratio, 0.95 [0.75‐1.20]) (Table 2). Cumulative mean events over time are given in Figure 2A,B. No trends with time are seen for nocturnal hypoglycaemia, but there were numerically more events at any time of day (24 hours) in the second 6‐month period with Gla‐300 compared with Gla‐100.
Table 2.
Population experiencing hypoglycaemia and event rates over 12 months
| Nocturnal hypoglycaemia (00:00–05:59 h) | Hypoglycaemia at any time (24 h) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gla‐300 (n = 274) | Gla‐100 (n = 275) | RR | 95% CI | Gla‐300 (n = 274) | Gla‐100 (n = 275) | RR | 95% CI | ||
| Total person‐years | 237.5 | 242.2 | 237.5 | 242.2 | |||||
| Confirmed or severe hypoglycaemia | |||||||||
| ≤3.9 mmol/L (≤70 mg/dL) | Participants, n (%) | 199 (72.6) | 205 (74.5) | 0.97 | 0.88–1.08 | 260 (94.9) | 260 (94.5) | 1.00 | 0.96–1.05 |
| Events, n (per person‐year) | 1917 (8.1) | 2079 (8.6) | 0.95 | 0.75–1.20 | 18 018 (75.9) | 16 661 (68.8) | 1.11 | 0.97–1.29 | |
| <3.0 mmol/L (<54 mg/dL) | Participants, n (%) | 151 (55.1) | 162 (58.9) | 0.94 | 0.81–1.08 | 225 (82.1) | 231 (84.0) | 0.98 | 0.91–1.06 |
| Events, n (per person‐year) | 658 (2.8) | 750 (3.1) | 0.90 | 0.68–1.20 | 4372 (18.4) | 4068 (16.8) | 1.11 | 0.91–1.35 | |
| Documented symptomatic hypoglycaemia | |||||||||
| ≤3.9 mmol/L (≤70 mg/dL) | Participants, n (%) | 176 (64.2) | 174 (63.3) | 1.01 | 0.89–1.15 | 240 (87.6) | 238 (86.5) | 1.01 | 0.95–1.08 |
| Events, n (per person‐year) | 1228 (5.2) | 1379 (5.7) | 0.91 | 0.69–1.21 | 9286 (39.1) | 8490 (35.1) | 1.12 | 0.92–1.37 | |
| <3.0 mmol/L (<54 mg/dL) | Participants, n (%) | 133 (48.5) | 134 (48.7) | 1.00 | 0.84–1.18 | 202 (73.7) | 206 (74.9) | 0.98 | 0.89–1.09 |
| Events, n (per person‐year) | 472 (2.0) | 574 (2.4) | 0.85 | 0.61–1.18 | 2522 (10.6) | 2559 (10.6) | 1.01 | 0.80–1.28 | |
| Severe hypoglycaemia | Participants, n (%) | 9 (3.3) | 9 (3.3) | 1.01 | 0.44–2.28 | 25 (9.1) | 31 (11.3) | 0.81 | 0.50–1.33 |
| Events, n (per person‐year) | 18 (0.08) | 11 (0.05) | 1.57 | 0.49–5.04 | 87 (0.37) | 57 (0.24) | 1.61 | 0.57–4.58 | |
Abbreviations: CI, confidence interval; RR, risk ratio for percentage of participants with ≥1 event, or rate ratio for events per person‐year.
Safety population.
Figure 2.
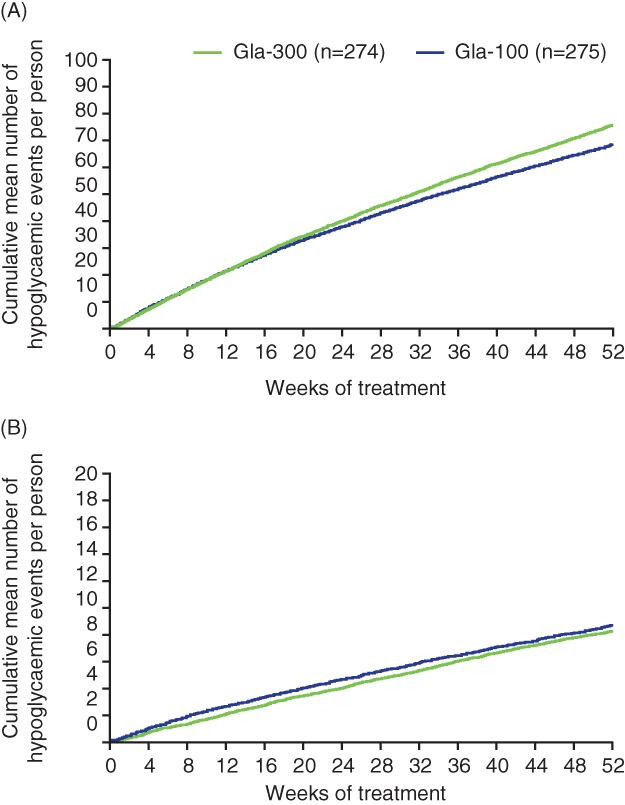
Cumulative mean number of confirmed (≤3.9 mmol/L [≤70 mg/dL]) or severe hypoglycaemic events per participant during 12 months of treatment with Gla‐300 or Gla‐100: A, events at any time of day (24 hours) and B, nocturnal events. Safety population
In the sensitivity analysis with a confirmation threshold of <3.0 mmol/L (<54 mg/dL), risk of hypoglycaemia and rate ratios remained statistically non‐significant (Table 2). Similar patterns were observed using a definition of documented symptomatic hypoglycaemia, at either plasma glucose threshold (Table 2). When hypoglycaemia rates were compared for the morning and evening groups statistical power was lower; thus, the central estimates were more erratic and confidence intervals were wider (Table S2). There were no statistically significant differences in any category of confirmed hypoglycaemia or documented symptomatic hypoglycaemia between study insulins.
At least 1 severe hypoglycaemic event at any time of day was reported by 25 (9%) participants in the Gla‐300 group and by 31 (11%) participants in the Gla‐100 group. Among these, 9 (3%) in each group experienced nocturnal events (Table 2). Annualized rates (events/person‐year) of severe hypoglycaemia were 0.37 for Gla‐300 and 0.24 for Gla‐100 at any time of day (24 hours), and 0.08 and 0.05, respectively, during the night. Confidence intervals for study prevalence and event rates were very wide (Table 2 and Table S2).
3.5. Body weight
Body weight increased in both treatment groups, but the statistically significant difference at 6 months in favour of Gla‐30010 was lost at 12 months (LS mean difference [95% CI] in change from baseline to month 12 [last on‐treatment value], −0.5 [−1.1 to 0.1] kg [P = .098]) (Figure 1B).
3.6. Participant‐reported outcomes
Mean change in total DTSQs scores from baseline to month 12 was similar in the 2 groups (Gla‐300, 1.3 [SD, 5.3]; Gla‐100, 1.6 [SD, 4.9]) (Table S3). Health status measured with EQ‐5D showed no change over 12 months in either group (Table S3). For HFS II scores, mean change from baseline to month 12 in total HFS II score was small and similar between groups (Gla‐300, −0.02 [SD, 0.40]; Gla‐100, −0.02 [SD, 0.43]) (Table S3).
3.7. Adverse events and insulin antibodies
The number of participants who reported an AE during the 12‐month treatment period was similar in the Gla‐300 (198 [72%]) and Gla‐100 (187 [68%]) groups, as was the pattern of AEs. Serious AEs were reported by 27 (10%) Gla‐300 participants and 26 (10%) Gla‐100 participants, without a notable difference for any type of event. One participant in the Gla‐300 group, with pre‐existing cardiovascular disease, died of a cardiac event during the first 6‐month period.10 Five participants in the Gla‐300 group and 4 in the Gla‐100 group withdrew from the study because of AEs. Injection site reactions, none serious or resulting in treatment discontinuation, were reported in 8 participants (2.9%) in the Gla‐300 group and 4 participants (1.5%) in the Gla‐100 group.
Throughout the 12‐month on‐treatment period, the percentage of participants who were positive at any time for anti‐insulin antibodies was comparable between the Gla‐300 (89% [237/266]) and the Gla‐100 (87% [235/270]) groups. The percentage of participants who were negative for anti‐insulin antibodies at baseline, but positive later, was also comparable (74% [72/97] and 74% [90/122], respectively).
4. DISCUSSION
Here we report on the 12‐month period of observation for the EDITION 4 study. This nearly doubles the exposure of people with T1DM to Gla‐300 as compared to the 6‐month data,10 while maintaining the primary randomization, both vs Gla‐100 and between morning and evening injections in both treatment groups. The report thus includes considerably more tolerability and safety data than did the primary 6‐month study.10 The extension‐period data have also helped to identify longer‐term trends in glucose control, and allow hypoglycaemia rates that are less influenced by distortions in the period after randomization that resulted from beginning a new insulin treatment with which the investigators and participants had no prior experience.
In the equivalent basal and meal‐time insulin study in T2DM, EDITION 1, evolution of blood glucose control in the second 6‐month period showed a small but statistically significant advantage of Gla‐300 in change in HbA1c from baseline at 12 months.8 In EDITION 4, the change from baseline in HbA1c observed at 6 months remained comparable between Gla‐300 and Gla‐100 throughout the 12‐month study period (Figure 1C and Table 1). This finding is in line with that observed at 12 months in the basal and meal‐time insulin study in T1DM conducted in Japan, EDITION JP 1.15 However, for HbA1c in the morning vs evening injection groups in EDITION 4, which for Gla‐100 was comparable both at 6 and 12 months, for Gla‐300 there was a significantly larger decrease in favour of the morning group (Figure 1D). It should be noted that the morning injection groups used higher (basal and total) insulin doses than the evening injection groups, for both Gla‐300 and Gla‐100. The reason for this is not clear, and comparison of morning and evening injection schedules (a specified objective secondary to the overall comparison of the 2 insulins) is complicated by diurnal hormonal changes and differences in patterns of physical activity. Meal‐time insulin use may also be a contributing factor, although in this study very little titration of meal‐time insulin dose occurred. Of interest, a study in T1DM using continuous glucose monitoring (CGM) showed that Gla‐300 24‐hour glucose profiles did not differ, irrespective of basal insulin being administered in the morning or evening16; a large ongoing study using CGM to investigate Gla‐300 and Gla‐100 given in the morning may provide further insights.17 The findings presented here highlight the value of longer‐term studies.
The 12‐month data from EDITION 4 showed that change in laboratory‐measured clinic FPG was comparable between the Gla‐300 and Gla‐100 groups. This measure, however, is not relevant to clinical practice as it requires people with T1DM to delay breakfast and breakfast insulin, something they are advised to avoid absolutely. Unfortunately, findings from the SMPG profiles are also unclear, in part because of baseline differences between the 2 treatment groups for the 3:00 am and pre‐breakfast glucose levels. However, endpoint findings at these times are similar (Figure 1G). There is some suggestion of separation between the Gla‐300 and Gla‐100 month‐12 SMPG profiles in the evening (bedtime) (Figure 1G), and this would seem to be confirmed by the data in Figure 1H, where SMPG profiles at 12 months for morning and evening injection are given. No separation of the 2 insulins at bedtime is seen for morning injection, while a separation can be perceived for evening injections in favour of Gla‐300. Speculatively, such a separation between insulins could be related to a failure of Gla‐100 to provide insulinization as effective as that of Gla‐300 in the hours immediately before the next injection, consistent with the comparative pharmacodynamic clamp data.7 As a caution, it should be noted that statistical testing of individual self‐monitored time points was not in the pre‐determined analysis plan, and profile interpretations are a matter of observation only.
The difficulty in interpreting morning and evening profiles illustrates a study limitation, namely, that the double comparison (two insulins, and morning versus evening) results in relatively small individual populations (~140 people); consequently, each show relatively variable glucose profiles (Figure 1), particularly for laboratory‐measured clinic FPG and SMPG profiles. That said, for Gla‐300, which is expected from pharmacokinetic and pharmacodynamic studies, and notably from a CGM study, to be a true ≥24‐hour insulin,7, 16, 18 there should be no difference in glucose‐related outcomes between morning and evening. Any trend in morning injection control being better than that with evening injections could be explained by the higher dose used by the morning group.
Hypoglycaemia risks and rates did not differ between the 2 study insulins in any of the measures analysed (nocturnal/any time of day, confirmed or severe/documented symptomatic, thresholds of ≤3.9 or <3.0 mmol/L, severe alone) (Table 2). However, as is usual in this type of trial, EDITION 4 was not powered to detect differences in hypoglycaemia. Numerical differences in anytime (24 hours) severe hypoglycaemia event rates, which do not approach statistical significance, can be explained by the fact that 1 participant in the Gla‐300 group reported 42 severe daytime events during the extension period. Cumulative event curves support the rate ratio data in revealing no differences between Gla‐300 and Gla‐100, but there is a trend to divergence of anytime events in the second 6‐month period, which needs further study (Figure 2). These findings differ from those of the EDITION JP 1 study, which investigated evening basal insulin injections; in that study Gla‐300 was associated with a lower risk and rate of nocturnal (0:00–05:59 am) confirmed (<3.0 mmol/L [<54 mg/dL]) or severe hypoglycaemia vs Gla‐100.15 They also differ from results with another long‐acting insulin (insulin degludec) in T1DM when given as an evening injection, which show lower rates of nocturnal hypoglycaemia with insulin degludec vs Gla‐100.19, 20 However, of note, a trial‐level meta‐analysis of degludec and Gla‐100 in T2DM suggests less reduction in HbA1c with degludec.21 When Gla‐300 is used in T2DM, an advantage over Gla‐100 was found, both in the basal plus meal‐time study to 1 year,8 and in the patient‐level meta‐analysis of 3 international studies in T2DM.22 In both the present study and the degludec studies, nocturnal hypoglycaemia rates remain clinically important even with the new insulins, emphasizing that flat true ≥24‐hour insulin profiles still suffer from lack of the minute‐to‐minute control of insulin delivery that is needed physiologically to eliminate the risk of hypoglycaemia.23
Reported AEs over 12 months were similar in the 2 groups; most would be background noise.10, 24 Data concerning serious AEs, whether overall or when analysed by organ and term (data not shown), did not reveal any signal of concern, and injection site reactions did not differ between treatment groups. As noted previously,10 the circulating active metabolite of glargine is the same for Gla‐300 as for the familiar and much‐tested Gla‐100.
The study limitations are generally inherent to clinical studies of this kind in people with T1DM. Confounding of glucose control and, particularly, hypoglycaemia may occur through the use and titration of meal‐time insulins, and while doses of these did not differ between treatment groups at 6 months, they were slightly different at 12 months (Figure 1A). This is relevant, as the majority of hypoglycaemic events occurred during the day rather than at night (Table 2 and Figure 2). HbA1c reduction was less in this study than that seen in a study of insulin degludec.20 It is not clear if this is related to the population studied or the insulin dose titration, but it may have limited statistical power to show differences in hypoglycaemia event rates. In addition, the discontinuation rate in this study was higher than is desirable, although this was matched between the Gla‐300 and Gla‐100 groups. While our study groups are perhaps typical of a modern T1DM population, the relatively high mean age and BMI, and the total insulin dose, may mean that the results are less generalizable to younger or leaner people.
In conclusion, at 12 months the results of the EDITION 4 clinical trial suggest that, in people with T1DM of long duration studied in a range of countries, Gla‐300 has glucose control properties similar to those of Gla‐100, albeit with a somewhat higher dose requirement. There was no evidence of new tolerability or safety issues over the 12 months of exposure. Comparison between morning and evening injection suggests little difference in glucose profiles and no difference in hypoglycaemia or AEs for Gla‐300, consistent with pharmacokinetic/pharmacodynamic data. These findings imply that the timing of Gla‐300 injection can be flexible, allowing injection at any time of day. More complete assessment of glucose profiles provided by the use of CGM may better determine the relative benefits of Gla‐300 over Gla‐100, and facilitate better management of meal‐time insulin in the context of a more stable basal insulin effect. Furthermore, studies in routine clinical practice may help evaluate the advantage to users of morning rather than evening injection of the basal insulin.
Supporting information
Figure S1. Participant study disposition.
Table S1. Change in basal, meal‐time and total insulin doses from baseline to month 12 by treatment group and time of injection (morning or evening).
Table S2. Hypoglycaemic events (events/person‐year) over 12 months, by treatment group and morning or evening injection time.
Table S3. Participant‐reported outcomes over 12 months of treatment, by treatment group.
ACKNOWLEDGEMENTS
The authors thank the study participants, trial staff and investigators for their participation. Editorial assistance was provided by Simon Rees, PhD, of Fishawack Communications, and was funded by Sanofi. Isabel Muehlen‐Bartmer of Sanofi contributed to the design and treatment considerations for the trial and was involved in the analysis and interpretation of the data.
Conflict of interest
P. D. H. or his affiliated institutions receive funding from AntriaBio, AstraZeneca, Biocon, GlaxoSmithKline, Hanmi, Janssen, Merck (MSD), Novo Nordisk, Roche Diagnostics and Sanofi. R. M. B. received research support from, served as a consultant for, or served on a scientific advisory board for Abbott Diabetes Care, Amylin, Bayer, Becton Dickinson, Boehringer Ingelheim, Bristol‐Myers Squibb‐AstraZeneca Alliance, Calibra, Dexcom, Eli Lilly, Halozyme, Hygieia, Johnson & Johnson, Medtronic, Merck, Novo Nordisk, Roche, Sanofi and Takeda. R. M. B.’s employer, nonprofit Park Nicollet Institute, contracts for his services and he receives no personal income. R. M. B. has inherited Merck stock. R. M. B. has been a volunteer for the American Diabetes Association and JDRF. G. B. B. received honoraria for advising and lecturing from Eli Lilly, Novartis and Sanofi. M. Z., M. R. and M. E. are employees of Sanofi. M. Z. and M. E. hold stocks/shares in Sanofi. M. C. R. received research grant support from AstraZeneca, Eli Lilly and Sanofi, and honoraria for consulting and/or speaking from AstraZeneca Alliance, Biodel, Elcelyx, Eli Lilly, GlaxoSmithKline, Sanofi, Theracos and Valeritas. M. C. R.’s dualities of interest have been reviewed and managed by Oregon Health and Science University. No other potential conflicts of interest relevant to this article were reported.
Author contributions
P. D. H., R. M. B., G. B. B. and M. C. R. contributed to protocol design, analysis and interpretation of the data, and writing the manuscript. M. Z., M. R. and M. E. contributed to the design and treatment considerations for the trial and were involved in the analysis and interpretation of data. All authors had full access to the study data and take final responsibility for submission of this manuscript for publication.
Home PD, Bergenstal RM, Bolli GB et al. Glycaemic control and hypoglycaemia during 12 months of randomized treatment with insulin glargine 300 U/mL versus glargine 100 U/mL in people with type 1 diabetes (EDITION 4). Diabetes Obes Metab. 2018;20:121–128. https://doi.org/10.1111/dom.13048
Funding information Sanofi was the sponsor, and coordinated the study (Clinical trial registration: NCT01683266, ClinicalTrials.gov), monitored clinical sites, collected and managed the data, and performed statistical analyses
REFERENCES
- 1. The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. [DOI] [PubMed] [Google Scholar]
- 2. Orchard TJ, Nathan DM, Zinman B, et al. Association between 7 years of intensive treatment of type 1 diabetes and long‐term mortality. JAMA. 2015;313:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Diabetes Association . (7) Approaches to glycemic treatment. Diabetes Care. 2015;38(suppl):S41–S48. [DOI] [PubMed] [Google Scholar]
- 4. Garber AJ. Will the next generation of basal insulins offer clinical advantages? Diabetes Obes Metab. 2014;16:483–491. [DOI] [PubMed] [Google Scholar]
- 5. Peyrot M, Barnett AH, Meneghini LF, Schumm‐Draeger PM. Insulin adherence behaviours and barriers in the multinational global attitudes of patients and physicians in insulin therapy study. Diabet Med. 2012;29:682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grunberger G. The need for better insulin therapy. Diabetes Obes Metab. 2013;15(suppl 1):1–5. [DOI] [PubMed] [Google Scholar]
- 7. Becker RH, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 Units·mL‐1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 Units·mL‐1. Diabetes Care. 2015;38:637–643. [DOI] [PubMed] [Google Scholar]
- 8. Riddle MC, Yki‐Jarvinen H, Bolli GB, et al. One‐year sustained glycaemic control and less hypoglycaemia with new insulin glargine 300 U/ml compared with 100 U/ml in people with type 2 diabetes using basal plus meal‐time insulin: the EDITION 1 12‐month randomized trial, including 6‐month extension. Diabetes Obes Metab. 2015;17:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yki‐Järvinen H, Bergenstal RM, Bolli GB, et al. Glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus insulin glargine 100 U/ml in people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs: the EDITION 2 randomized 12‐month trial including 6‐month extension. Diabetes Obes Metab. 2015;17:1142–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Home PD, Bergenstal RM, Bolli GB, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 1 diabetes: a randomized, phase 3a, open‐label clinical trial (EDITION 4). Diabetes Care. 2015;38:2217–2225. [DOI] [PubMed] [Google Scholar]
- 11. American Diabetes Association Workgroup on Hypoglycemia. Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28:1245–1249. [DOI] [PubMed] [Google Scholar]
- 12. Lewis KS, Bradley C, Knight G, Boulton AJ, Ward JD. A measure of treatment satisfaction designed specifically for people with insulin‐dependent diabetes. Diabet Med. 1988;5:235–242. [DOI] [PubMed] [Google Scholar]
- 13. EuroQol‐‐a new facility for the measurement of health‐related quality of life . Health Policy. 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 14. Irvine A, Cox DJ, Gonder‐Frederick L. The fear of hypoglycemia scale In: Bradley C, ed. Handbook of Psychology and Diabetes: A Guide to Psychological Measurement and Diabetes Research and Practice. Hove, UK: Psychology Press; 1994:133–155. [Google Scholar]
- 15. Matsuhisa M, Koyama M, Cheng X, et al. Sustained glycaemic control and less nocturnal hypoglycaemia with insulin glargine 300U/mL compared with glargine 100U/mL in Japanese adults with type 1 diabetes (EDITION JP 1 randomised 12‐month trial including 6‐month extension). Diabetes Res Clin Pract. 2016;122:133–140. [DOI] [PubMed] [Google Scholar]
- 16. Bergenstal RM, Bailey TS, Rodbard D, et al. Comparison of insulin glargine 300 U/mL and 100 U/mL in adults with type 1 diabetes: continuous glucose monitoring profiles and variability using morning or evening injections. Diabetes Care. 2017;40:554–560. [DOI] [PubMed] [Google Scholar]
- 17. Sanofi . Randomized, active‐controlled, parallel group, 16‐week open label study comparing the efficacy and safety of the morning injection of Toujeo (insulin glargine‐U300) versus lantus in patients with type 1 diabetes mellitus. https://clinicaltrials.gov/ct2/show/NCT02688933. Accessed February 17, 2017.
- 18. Shiramoto M, Eto T, Irie S, et al. Single‐dose new insulin glargine 300 U/ml provides prolonged, stable glycaemic control in Japanese and European people with type 1 diabetes. Diabetes Obes Metab. 2015;17:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bode BW, Buse JB, Fisher M, et al. Insulin degludec improves glycaemic control with lower nocturnal hypoglycaemia risk than insulin glargine in basal‐bolus treatment with mealtime insulin aspart in Type 1 diabetes (BEGIN® Basal–Bolus Type 1): 2‐year results of a randomized clinical trial. Diabetic Med. 2013;30:1293–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lane W, Bailey TS, Gerety G, et al. SWITCH 1: reduced risk of hypoglycaemia with insulin degludec vs insulin glargine U100 in patients with type 1 diabetes: a randomised, double‐blind, crossover trial. Diabetologia. 2016;59(suppl 1):1.27539147 [Google Scholar]
- 21. Roussel R, Ritzel R, Chevalier S, Balkau B, Rosenstock J. Clinical perspectives from the BEGIN and EDITION programmes: trial‐level meta‐analyses outcomes with either degludec or glargine 300 U/ml vs glargine 100 U/ml in T2DM. Diabetologia. 2016;59(suppl 1):1.27539147 [Google Scholar]
- 22. Ritzel R, Roussel R, Bolli GB, et al. Patient‐level meta‐analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes. Diabetes Obes Metab. 2015;17:859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Home PD. Plasma insulin profiles after subcutaneous injection: how close can we get to physiology in people with diabetes? Diabetes Obes Metab. 2015;17:1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tan K, Petrie KJ, Faasse K, Bolland MJ, Grey A. Unhelpful information about adverse drug reactions. BMJ. 2014;349:g5019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Participant study disposition.
Table S1. Change in basal, meal‐time and total insulin doses from baseline to month 12 by treatment group and time of injection (morning or evening).
Table S2. Hypoglycaemic events (events/person‐year) over 12 months, by treatment group and morning or evening injection time.
Table S3. Participant‐reported outcomes over 12 months of treatment, by treatment group.


