Figure 1.
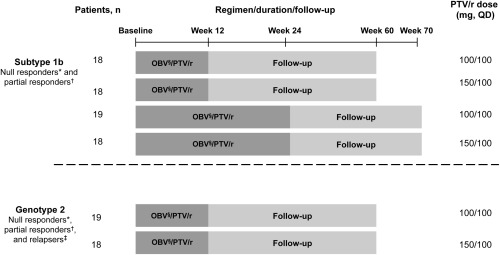
Study design. *Patients not achieving a 2 log10 IU/mL reduction in HCV RNA at week 12 after ≥10 weeks of pegIFN/RBV. †Patients who achieved a ≥2 log10 IU/mL reduction in HCV RNA at week 12 after ≥20 weeks of pegIFN/RBV but had HCV RNA levels above the lower limit of detection at treatment end. ‡Patients with undetectable levels of HCV RNA after one or more courses of pegIFN/RBV treatment who had detectable HCV RNA within 24 weeks. §Dose: 25 mg QD. Abbreviations: OBV, ombitasvir; PTV, paritaprevir; QD, once daily; r, ritonavir.
