Figure 1.
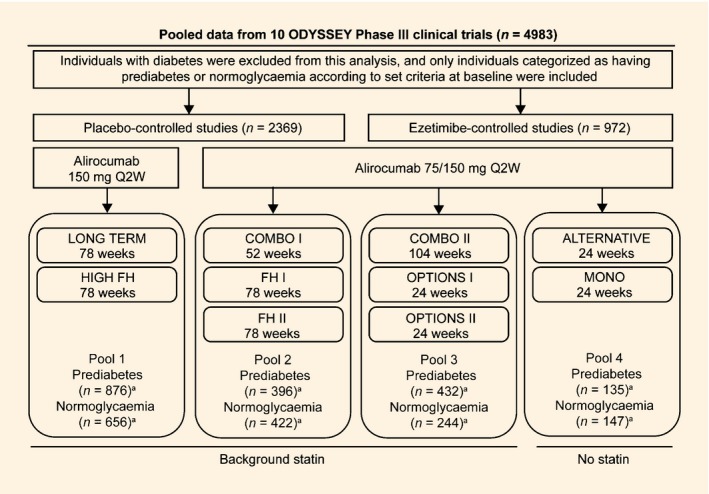
Overview of ODYSSEY trials included in this analysis (randomized population). FH, familial hypercholesterolaemia; HeFH, heterozygous familial hypercholesterolaemia; Q2W, every 2 weeks. aITT population. The FH I, FH II and HIGH FH studies recruited only individuals with HeFH. COMBO I and COMBO II recruited individuals with non‐FH at high cardiovascular risk. OPTIONS I, OPTIONS II and LONG TERM recruited both individuals with HeFH and non‐FH. ALTERNATIVE recruited individuals with moderate to high cardiovascular risk and not receiving a statin due to intolerance. Individuals recruited in the MONO study had moderate cardiovascular risk not receiving statins or other lipid‐lowering therapy. Further details of the ODYSSEY trials included in this analysis have been published previously: Clinicaltrials.gov identifiers: ALTERNATIVE, NCT01709513 11; COMBO I, NCT01644175 10; COMBO II, NCT01644188 13; FH I, NCT01623115; FH II, NCT01709500 9; HIGH FH, NCT01617655 23; LONG TERM, NCT01507831 12; MONO, NCT01644474 14; OPTIONS I, NCT01730040 7; OPTIONS II, NCT01730053 8.
