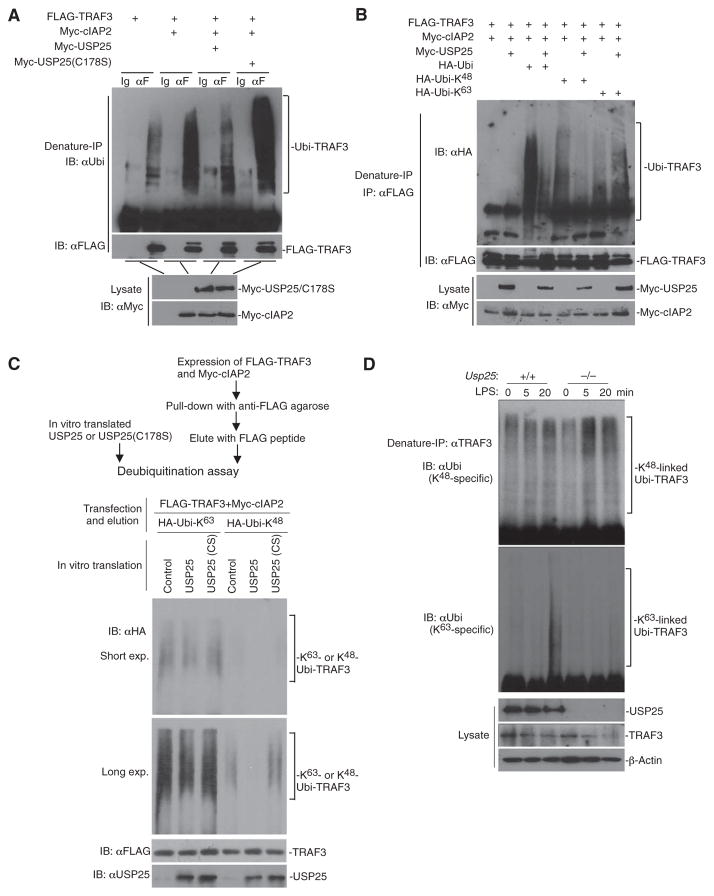
Fig. 6. USP25 cleaves the cIAP2-mediated K48-linked ubiquitin chain from TRAF3.
(A and B) USP25 cleaves K48-linked ubiquitin chains from TRAF3. HEK 293T cells were transfected with plasmids encoding the indicated constructs. Twenty hours later, cells were lysed and the cell lysates were denatured in 1% SDS and then heated to 95°C for 5 min. The denatured lysates were subjected to immunoprecipitation (denature-IP) with the control IgG (Ig) or an anti-FLAG antibody (αF). The immunoprecipitates were analyzed by Western blotting with antibodies against (A) ubiquitin (αUbi, top blot), (B) hemagglutinin (HA; αHA, top blot), or (A and B) FLAG (middle blots). The abundances of the indicated proteins in the cell lysates were determined by Western blotting analysis with an anti-Myc antibody (αMyc, bottom blots). (C) USP25 specifically cleaves K63-linked ubiquitin chains from TRAF3 in vitro. The strategy to obtain TRAF3 protein that was modified with either K63- or K48-linked ubiquitin chains and then combine them with USP25 or USP25(C178S) proteins is illustrated (also see Materials and Methods for further details). A deubiquitination assay was performed by mixing the indicated proteins followed by Western blotting analysis with antibodies against HA, FLAG, or USP25. exp., exposure. (D) Deficiency in USP25 results in increased K48-linked ubiquitination of TRAF3 after stimulation with LPS. WT and Usp25−/− BMDMs were treated with LPS (10 μg/ml) for the indicated times. The cells were lysed, and the cell lysates were subjected to denaturing IP with an anti-TRAF3 antibody. The immunoprecipitated samples were subjected to Western blotting analysis with antibodies specific for K48- or K63-linked ubiquitin (top two blots). The abundances of the indicated proteins in the cell lysates were determined by Western blotting analysis with specific antibodies against the indicated proteins (bottom blot). Data are representative of three (A and B) or two (C and D) independent experiments.